Carcinoma of the Breast
The Common Vein
Copyright 2009
Niharika Dixit MD Ashley Davidoff MD
Definition
Breast cancer is a malignant condition of the ductal or glandular epithelium. The causes for the disease are multifactorial and include genetic, environmental,physical (body habitus) and obstetric elements, but 75% of patients have no risk factors except for gender and age. The disease starts out locally as an aberrant cell in the epithelium, grows and multiplies into a nodule or mass and subsequently results in local extension. When the cancer has not yet invaded it is called carcinoma in situ and when the epithelial membrane is breached it is considered truly malignant and invasive. Each tummor has a specific biology. Sometimes the cells are well differentiated, suggesting a lower grade malignancy, some are intermediate, while others are categorized as high grade and poorly differentiated, suggesting a more aggressive disease. the cells also express individual by protein and gene expression characterized best by immunohistochemistry.
The disease is complicated by metastatic disease to regional lymph nodes and distant organs. TNM (status of the tumor,nodes, and metastases)classification is commonly used
The diagnosis may be suspected clinically on finding a new mass in the breast, new nipple or skin retraction, but mammography and MRI are utilized to suggest the diagnosis, and percutaneous biopsy is used to confirm the diagnosis.
The mainstay of treatment is surgery when the tumor is considered localized, usually combined with chemotherapy and radiation therapy. With optimal treatment 10 year disease free survival ranges from 10-98%, depending on type, grade and staging.
 Breast Carcinoma Breast Carcinoma
Artistic Rendition |
| This artistic rendition of a mammogram shows the crab like malignancy (red) invading the breast tissue.
27993b02.62k.8s breast cancer spiculated lesion infiltrating carcinoma space occupation invasion Davidoff art all rights resrved copyright 20 |
Overview
Breast cancer is the second most common cancer in the world after lung cancer. Although it is 100 times more common in woman, it affects males as well. It constitutes about 10.9 % of all newly diagnosed cancers each year.1 Breast cancer is the most frequently diagnosed cancer in women in the United States, accounting for an estimated 274,900 new cases (212,920 invasive cancers and 61,980 in situ carcinomas) and 40,970 deaths in 2006. In the United States, the age-specific incidence of breast cancer increases with age, to a lifetime risk of breast cancer of 1 in 8 (to 110 years of age); by age 40, approximately 1 in 250 women will have been diagnosed with breast cancer annually; at 60 years of age, the figure is 1 in 35 women. Breast cancer diagnosis is devastating to any woman; however tremendous strides have been made in screening, treatment and follow up of the disease which has made breast cancer a very treatable disease.
History of the Disease
Breast is likely to be one of the first known cancers, likely because of its external manifestations.. It dates back to 1600 BC in ancient Egypt twhere both recognition of the disease and its treatment begins with cauterization of tumors with an instrument known as “the fire drill.” Although medical practitioners were able to remove these tumors in some cases, they were for the most part unable to stop the disease from taking its course. Writes the ancient Egyptian doctor of breast cancer: “There is no treatment.”
Such was the prevailing wisdom throughout the history of breast cancer. Although cases of breast cancer are documented throughout history, it is not until the 17th century in that European doctors linked the tumors in the breast to the lymph glands in the armpit. In the early 18th century two extremely important figures in the history of breast cancer arose: Jean Louis Petit and Benjamin Bell who performed the first surgeries removing the lymph nodes, breast tissue and breast muscle in order to remove the cancer from the body and fight its spread.
This pioneering work was continued by the surgeon William Stewart Halsted who began performing complete mastectomies in 1882. His procedure is known as the Halsted radical mastectomy, and was popular throughout the 20th century up until the 1970’s and is still common today.
While breast cancer survival rates have gone up somewhat in the last twenty years, the fact remains that this disease is still far too prevalent and resulting in the death of far too many women.
Many great strides have been made in the history of breast cancer treatment but there is still a great amount of work to be done. One in 12 or 13 women will suffer from breast cancer at some time during their lives in the western world. In the United States these numbers can be even higher. One of the most important aspects of the fight against breast cancer is breast cancer awareness. The more knowledge known about the disease and the more done to check for early warning signs, the better is the chance for survival. Continuing research and breakthrough in treatment are a part of the ongoing history of breast cancer
Causes and Predisposing Factors
Genetic:
A personal or family history of the disease, (in mothers or fathers family) particularly if acquired before the age of 40, increasingly raises the risk of the disease. Breast cancer is approximately twice as common among first-degree relatives of breast cancer patients as in those women with no family history of the disease. The two most important breast cancer susceptibility genes, BRCA1 and BRCA2, were identified by linkage analyses in the mid-1990s. Since then, rare germ line mutations in several other genes, such as TP53, PTEN, and ATM, have been shown to confer an increased risk of breast cancer. BRCA1 and BRCA2 account for less than 20% of familial clustering of breast cancer. It is likely that most familial breast cancers have polygenic transmission, where multiple genes are involved and difficult to identify.2
It is more common in Caucasian women, than Asian or African American women. The Jewish Ashkenazi population displays a 10 fold higher frequency of 2 founder mutations (185delAG and 5382insC) therefore screening of such mutations in the above population is indicated.
Reproductive and endocrine risk factors:
Gender is the most important risk factor in the development of breast cancer, which occurs 100 times more frequently in women than in men. This is due to endocrine factors that relate to lifetime exposure to estrogen. Estrogen is itimately involved in every aspect of female reproducrtive physiology including normal sexual development, regulation of menstrual cycles, tand , pregnancy. The longer the exposure to estrogen the greater the risk of pregnancy. Therefore the older the patient the greater the exposure to estrogen and the higher the risk. Additionally, early menarche and late menopause raise the risk by increasing the time of estrogen exposure ( > 30 years menstrual time). On the other hand, diets low in fat and high in fiber decrease the levels of estrogen and lower the risk of breast carcinoma. Age is also an important risk factor for the development of breast cancer, since in essence translates to years of exposure to estrogen.
Protective factors include, longer lactation periods, and moderate levels of exercise. There is some evidence that circulating levels of estrogen are lower in women who excercise. In addition they have less body fat.
Birth Control Pills and Hormone Replacement Therapy
The effect of birth control pills is controversial. Theoretically it should depend on the concentration of estrogen in the pill. The earlier birth control pills had higher levels of estrogen compared to the current OCP’s, and this may relate to the association.
Estrogen in hormone replacement therapy (HRT) in post menopausal women, has been linked to increased risk of both breast and endometrial cancer. As such, there has been a move to recommend discontinuation of HRT in post menopausal women.
Obesity:
There appears to be an association between body mass index (BMI) and breast cancer, which may be accounted for by increased estrogen levels in women with a higher BMI. The BMI is a ratio of weight to height. Obesity after menopause is especially important since the ovaries at this time stop producing estrogen, and if estrogen from fat is still present then it increases lifetime exposure.
Diet:
Alcohol consumption is the best-established dietary factor associated with increased risk for breast cancer, and seems to be related to the amount of alcohol consumed. A proposed explanation is that alcohol increases the amount of circulating estrogen.
Dietary phytoestrogens, are estrogens found in vegetables. They serve as a protective factor since they are much weaker than endogenous estrogen, but compete for estrogen receptors, and thus reduce the effect of the endogenous estrogen theoretically resulting in less cell division.
Ionizing radiations:
Data about risk in women from radiation is derived mainly from epidemiologic studies of patients exposed to diagnostic or therapeutic radiation. Low-dose radiation exposure to the breast is carcinogenic, and risk increases as a linear function of increasing dose. The risk is particularly high in the developing breast (age <20).
Geographic Distribution
The incidence of breast cancer varies significantly among differennt countries, and is highest in Northern European countries and in the United States, intermediate in Southern America and Southern and Eastern Europe, and lowest in Asia (Japan, Singapore and urban China have seen a rise in rates with the advent of Western-style economy). Breast cancer incidence and mortality vary sigificatly within the United States. The incidence of breast cancer appears to be highest in white women from Hawaii (128:100,000) followed by those from San Fransisco and the Northeast.The lowest incidence is found in Utah (98:100,000) and New Mexico
The variation of incidence for African-American woment is relatively small ( 94-106:100,000).
The interaction of many different risk factors in the initiation of breast cancer has lead to the creation and use of risk-assessment models. This helps predict the risk of management and determine the best course of action. The most frequently used model was designed by Gail in 1970 and incorporates age at menarche, number of breast biopsies, age at first live birth and the number of first degree relatives with breast cancer. It is used to predict the risk of developing breast cancer per decade of life. One of the disadvantages of this model is that it doesn’t take into account age at which a family member was diagnosed and whether the disease occurred in both breasts — early onset and bilateral disease suggesting a BRCA mutation carrier
Structural Principles and Changes
Breast tissue consists of glandular, fatty and connective tissue. Each breast overlies the pectoral muscle of chest wall and is tethered to chest wall with tiny connective tissue fibers call ligaments of Cooper. Each breast is divided into about 15-20 lobes which are formed by smaller units called lobules. There are about 20-40 lobules per lobe. The lobules are made from small glandular structures called acini. There are 10-100 acini per lobule. A branching ductal system transports the milk from the acicini into the lobular ducts, which converge to a single lactiferous duct, which brings the milk to the nipple.
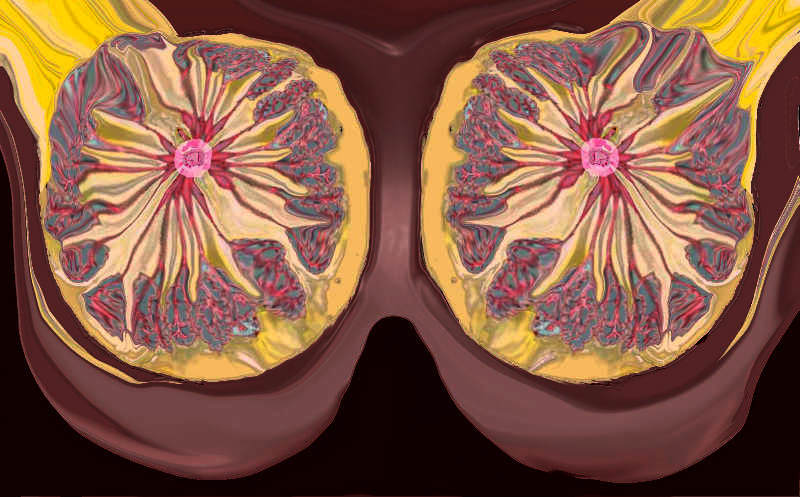
 Components of the Breast Components of the Breast |
| This CT is from a patient with normal breasts by CT scan. The image on the left shows the CTscan without overlay through the mid chest, and the image on the right shows the parts of the breast overlaid in color to exemplify the parts. The nipple, surrounded by the areola is overlaid in dark pink. The triangular shaped glandular tissue is overlaid in lightpink and is surrrounded anteriorly by the superficial layer of fat, (light yellow), and the deep layer of fat posteriorly (dark yellow). Deep to the deep layer of fat is the chest wall that includes the pectoralis muscle and ribs.
The make up of the breast is extremely varied among patients with some breasts dominated by fat and others dominated by glandular tissue, and yet otyher dominanted fy connective tissue. In this patient fatty tissue dominates.
Courtesy Ashley Davidoff MD copyright 2009 all rights reserved 25483c.8s |
The Terminal Duct Lobular Unit (TDLU)
The understanding of cancer of the breast requires an understanding of the terminal duct lobular unit (TDLU). In order to understand the terminal duct lobular unit the histology of the breast needs to be understood.
Definition
The terminal duct lobular unit is the functional unit of the breast and is responsible for the production and transport milk to the lactiferous duct.
Structurally, it consists of a lobule of the breast with an extraobular terminal duct. A group of acini group together with an intralobular duct form a lobule, and the lobule empties into an extralobular duct. The TDLU is surrounded by fibrofatty connective tissue. 20-40 TDLU’s combine to form a lobe.
The TDLU is extremely sensitive to hormonal influences, and its appearance varies based on physiologic conditions. It changes within each cycle, enlarges significantly during pregnancy and lactation and finally atrophies in the post menopausal period. Because of its sensitivity to hormonal conditions, it is also subject to aberrances in the hormonal environment.
From a disease standpoint, the TDLU is the site of origin of ductal carcinoma in situ (DCIS), lobular carcinoma in situ, invasive ductal carcinoma, invasive lobular carcinoma, as well as more benign conditions such as fibroadenoma, fibrocystic disease, cysts, apocine metaplasia, adenosis and epitheliosis.
The diagnosis of disease in the TDLU is usually at a pathological level, since in general imaging techniques are not sensitive to the histological subtypes.
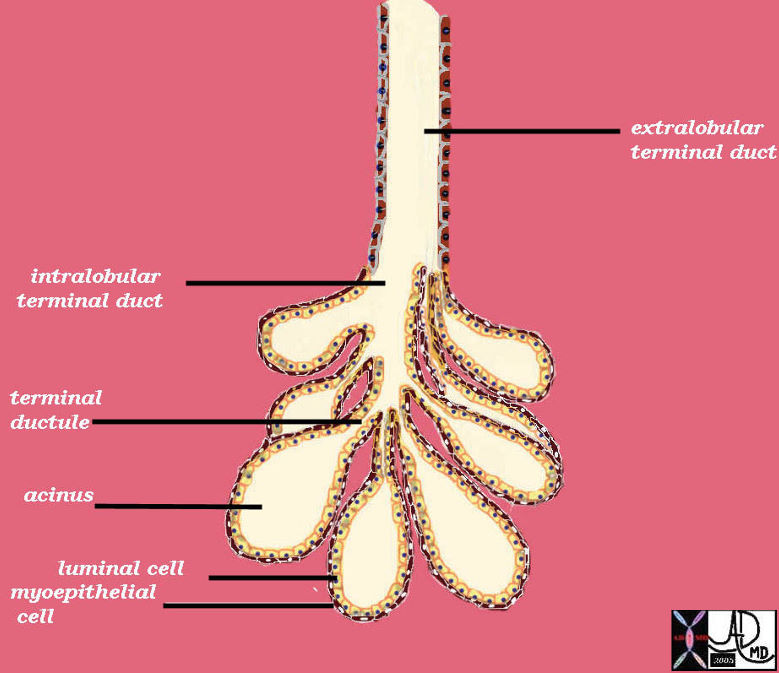
Terminal Duct Lobular Unit
Lobule of the Breast at a Histological Level
|
| The TDLU consists of a clump of grape like acini with their respective terminal ductules, all of which empty into an intralobular duct to form a lobule. The lobule empties into an extralobular duct. The extralobular duct and the lobule form the terminal duct lobular unit (TDLU). The acinus is two layers thick. The inner layer is the secretory epithelium of a cuboidal nature and the outer layer is a myoepithelial layer.
42707b03b45b05 breast mammary gland ducts ductules extralobular terminal duct intralobular terminal duct terminal ductule acini acinus alveoli alveolus surface epithelium luminal cell myoepithelial cell lobule terminal ductal lobular unit TDLU drawing anatomy histology normal Courtesy Ashley Davidoff MD |
The following section is a review of the applied histology of the breast, and provides a framework of how to understand the various subtypes of carcinoma.
Applied Histology of the Breast
The10-20 lobes of the breast are arranged radially around the breast and the single duct that subtends the lobe, empties independantly on the nipple.
Radially Arranged Lobules Each with a Single Duct Opening Onto the Nipple
|
| The lobules are arranged around the breast in radial fashion, just like the hands on a clock. In this artistic rendition the lobules are seen in teal blue, the acini within the lobules are seen as branching dark pink structures within the lobe. From each lobe, a single duct arises, and opens onto the nipple.
42657b07_800 Courtesy Ashley Davidoff MD breast lobule duct nipple fat glands glandular tissue normal anatomy drawing art Davidoff art |
Anatomical Sites and Subsites
1. Nipple
2. Central portion
3. Upper-inner quadrant
4. Lower-inner quadrant
5. Upper-outer quadrant
6. Lower-outer quadrant
7. Axillary tail
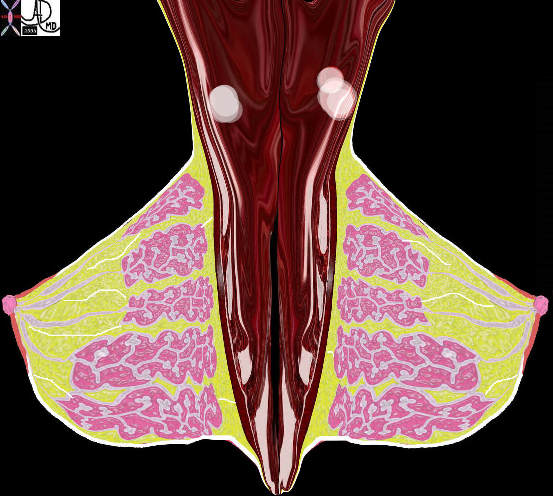
Lateral View of Both Breasts Showing Lobules
|
| The lateral view of both breasts against their respective chest walls show the lobules as pink structures within which reside the glandular elements (branching pale pink). The duct subtending the lobule enters the nipple. There are fine white strands of connective tissue that anchor the breast tissue to the skin in front , to other lobes, and the chest wall behind. These are called Cooper’s ligaments.
42707b03b19b breast mammary gland nipple areola ducts Coopers ligaments Cooper’s ligaments pectoralis muscle chest wall adipose tissue layers fat superficial adipose tissue deep retromammary interlobular fat adipose tissue stroma connective tissue parenchyma glandular apparatus mammary apparatus axilla axillary lymph node normal anatomy drawing Courtesy Ashley Davidoff MD |
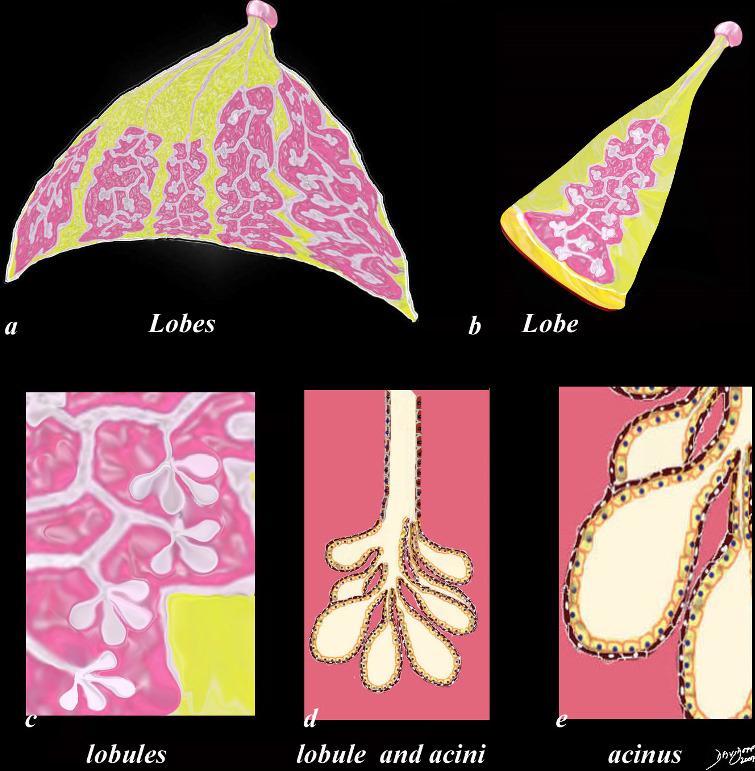
Glandular Makeup of the Breast
|
| The first drawing in a transverse plane (a) shows 4 of the 10 -20 lobes that make up the glandular tissue of the breast, each with their own lactiferous duct that enters the nipple. The second drawing (b) shows a single lobe made up of 20-40 lobules that look like white clumps of grapes on a stalk. The lobules shown in (c) are composed of ovoid sacs or tear drop sacs called acini. Only 3-4 are shown per small stalk (ductule) but there are between 10-100 per “bunch”. Image d is a high powered view of a lobule (the described bunch) and each of the tear dropped shaped “grapes” is called an acinus. or alveolus. Each acinus is an empty sac surrounded by two layers of epithelium. A high powered view of the inner lining (e) is shown consisting of two cell layers. The inner (luminal) layer is cuboidal and is responsible for milk secretion, and the outer lining consists of smooth muscle cells that enable the glands to contract and move the milk out of the acinus and into the ductal system.
code breast normal anatomy histology lobe lobules acini alveoli acinus lactiferous duct extralobular ductule intralobular ductule Davidoff art Courtesy Ashley Davidoff MD copyright 2009 all rights reserved
code breast normal anatomy histology lobe lobules acini alveoli acinus lactiferous duct extralobular ductule intralobular ductule Davidoff art Courtesy Ashley DAvidoff Md copyright 2009 all rights reserved 42707c29.8s |
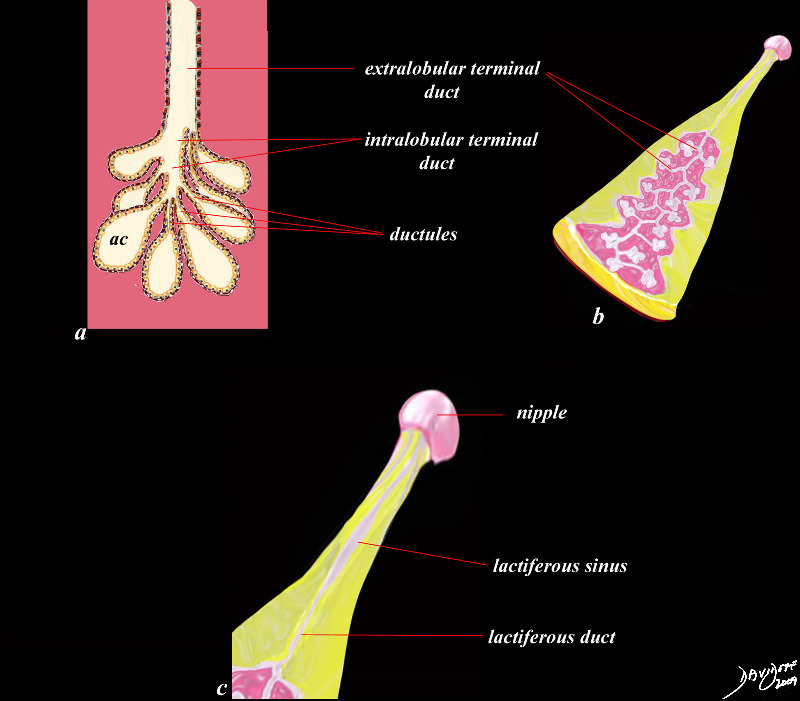
Ductal System of the Breast
|
| This diagram shows the ductal system of the breast. As the milk is secreted by the cells into the acini (ac) its transport is facilitated by smooth muscle contraction into the ductules subtending the acini. Multiple ductules join to form an intralobular terminal duct which advances outside of the lobule into an extralobular terminal duct. This duct, that subserves a single clump of acini, converges with 20-40 other extralobular ducts to form the main duct of the lobe and becomes the lactiferous duct (b). The lactiferous duct (c) has a small fusiform dilatation just before the nipple called the lactiferous sinus which is able to distend, and store milk temporarily before it is needed and expelled.
code breast ducts acinus acini lobule ductules terminal duct intralobular terminal duct extralobular terminal duct lactiferous duct lactiferous sinus Courtesy Ashley Davidoff MD Davidoff art copyright 2009 all rights reserved 42707b03b45b05c01.8s |
 Microscopic Histology of the Lobule Microscopic Histology of the Lobule |
| This histological section shows a terminal duct lobule unit. (TDLU) A lobule is ringed in black and the extralobular terminal duct is noted and labelled. The acini are shown ringed in red but the draining intralobular ducts are not seen The lobules and extralobular terminal ducts join to form a single lactiferous duct which exits at the nipple
code breast histology fat glands connective tissue extralobular ductule lobule acinus acini breast mammary glands ducts fat CourtesyFrank Reale MD copyright 2009 all rights reserved 13511b01c04.8s |
Bands of connective tissue, the suspensory ligaments, extend from the interlobular connective tissue to attach to the dermis.
Malignant breast disease
The carcinomas are the most significant lesions of the breast. There are two major types of breast carcinoma that arise from the lobule namely ductal carcinoma and lobular carcinoma. They both arise from the TDLU.
Carcinoma of the breast at this level is divided into the non invasive or “in situ” carcinomas on the one hand and the invasive carcinomas on the other. The “in situ” carcinomas are restricted to the lobule and do not show invasion of the basement membrane while in the invasive group, there is obvious transgression of the basement membrane.
The “in situ” group has variable behavioral patterns and outcomes. These lesions may remain dormant for a lifetime, or may even involute. They do however place the woman at increased risk of developing cancer and should therefore be treated with heightened awareness.
The two types of “in situ” carcinomas are called ductal carcinoma in situ (DCIS), and lobular carcinoma in situ (LCIS), and they are very different. DCIS is common and occurs in 70-95% of women at autopsy and is commonly associated with microcalcifications on mammography. It arises from ductal epithelium. LCIS is less common, clinically and radiologically more elusive, occurs in 1-5% of biopsies, and is rarely associated with microcalcifications on mammography. Once LCIS is identified the risk of developing cancer if untreated is about 30% within 20 years of diagnosis. It is not considered a precancerous disease, but a marker of increased risk.
The two more common invasive carcinomas of the breast are correspondingly called invasive ductal carcinoma and invasive lobular carcinoma and are defined by the transgression of the basement membrane by malignant cells.
The most common site of involvement is the upper outer quadrant since it is the location of “extra” tissue called the axillary tail of Spence. The presence of a larger amount of glandular tissue raises the relative incidence of disease in this quadrant. About 45% of carcinomas arise from this upper outer quadrant. Invasive ductal carcinoma is the most common type of carcinoma.
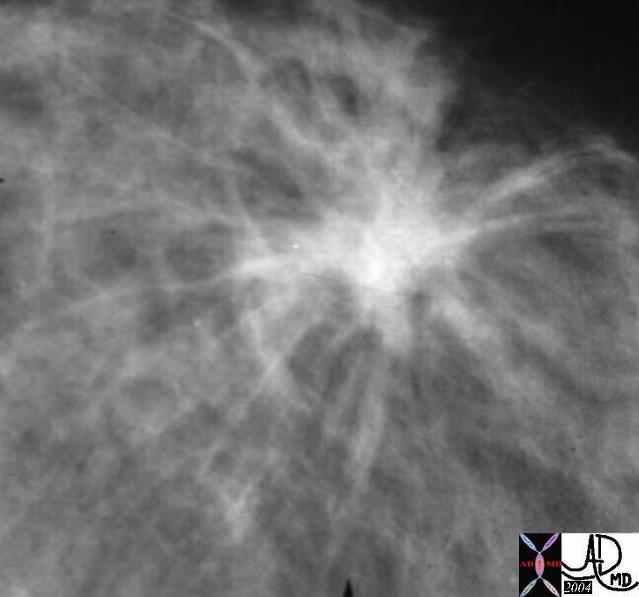 Spiculated Carcinoma Spiculated Carcinoma |
| The appearance of carcinoma on a mammogram can take many forms, one of which is shown above and characterized by its spiculated shape.
13523 breast + fx mass fx spiculated dx carcinoma mammogram mammography Courtesy Carl D’Orsi MD DB |
The current favored hypothesis on the evolution of breast cancer describes a progression similar to that in colon cancer ( Knudson’s hypothesis) that includes usual ductal hyperplasia, atypical hyperplasia, carcinoma in situ ( ductal carcinoma in situ and lobular carcinoma in situ) and invasive carcinoma.
Carcinoma in Situ
Each type of breast carcinoma in situ (DCIS and LCIS) has a distinct clinical and biological behavior, and they are discussed separately.
Ductal Carcinoma in Situ (DCIS)
DCIS is a stage zero malignancy of cells confined to the ducts or lobules by the basement membrane. Structurally the disease is characterized by malignant proliferation of epithelial cells cells within the ducts and lobules, with intact myoepithelial cells and basement membrane. The cells are able to spread through the ductal system and lobules, and therefore a single clone of cells can spread within the lobular system almost like sludge or debris in a river with intact river banks. DCIS is the most common type of non invasive cancer of the breast.
Histologically there are 5 architectural types including comedocarcinoma, solid, cribriform, papillary, and micropapillary. The comedocarcinoma shows the highest grade of behaviour, with the most malignant characteristics. There is associated necrosis of the cells giving rise to a cheesy material that can be squeezed like toothpaste out of the ducts. This necrotic debris undergoes dystrophic calcification which manifests as clusters of tubular branching microcalcifications on the mammogram.
Ductal carcinoma in situ (DCIS), also known as intraductal carcinoma, and is found in approximately 7% of all biopsied specimens. It is considered a premalignant condition and risk of invasive breast cancer is increased nearly five times in women with DCIS. Such risk is primarily ipsilateral as opposed to LCIS which is more likely to be multifocal and bilateral. Multicentricity is seen in 60-90% of patients with LCIS and in 40-80% of patients with DCIS. LCIS occurs bilaterally in 10-20% of DCIS cases and in 50-70% of LCIS cases.
Before mammography DCIS was primarily diagnosed by physical examination as a palpable lesion. In situ breast cancers constituted approximately 2% of all breast cancers in 1980 and LCIS was more common than DCIS by a ratio of 2:1. Screening mammography revealed a 14 fold increase in the incidence of in situ cancer (45%) and DCIS was diagnosed more frequently than LCIS, mostly because small non specific calcifications associated with DCIS are identifiable, whereas not a characteristic feature of LCIS.
The calcifications seen on mammography arise in the extralobular ducts, and hence tend to be small , clustered, heterogeneous but often rod shaped.
Biopsy of suspicious areas of calcification confirms the diagnosis.
Treatment in general includes excision of the lesion(s) and radiation therapy is often included. However the treatment is individualised taking factors such as age, multicentricity, state of health, and family history into account.
Prognosis is excellent with almost 100% cure rate.
Lobular Carcinoma in Situ (LCIS)
Lobular carcinoma is not truly a carcinoma, but has the risk of developing into a carcinoma. LCIS is considered a marker of increased risk for invasive breast cancer rather a premalignant lesion . Like DCIS, it can be considered a stage zero carcinoma, and malignant appearing cells are seen microscopically within the lobules of the breast, with a basement membrane that is intact, ie not invaded. The complicating statistic is that about 25% of patients with LCIS will develop invasive cancer in their lifetime, either from the lobules or from the ducts.
Lobular carcinoma in situ (LCIS) was first described in 1941 ( Foote and Stewart) and is characterized by three important features:
1) the lesion is a microscopic finding that is not apparent clinically and is usually an incidental finding;
2) the lesion is multicentric in the breast and often bilateral (30%) and
3) both infiltrating ductal and lobular carcinomas may develop after LCIS.
Lobular carcinoma in situ arises from the terminal lobular duct units. LCIS is a risk factor for bilateral breast cancer (approximately 1% per year) and therefore careful clinical followup is appropriate for most women.
Treatment is individualised but ranges from increased surveillance with frequent physical exams per year and mammograms once or twice per year. Tamoxifen reduces the risk of breast cancer ( by approximately 55%)
Bilateral mastectomy is not commonly performed, but patients with a strong family history, or who have unfavorable genetic mutations sometimes choose this option.
Statistics
LCIS lacks clinical and mammographic signs and therefore it’s true incidence is not known. Numerous authors have reviewed breast biopsies in an effort to determine the incidence of LCIS and the results varied from 0.5% ( Page et al) to 3.6% ( Haagensen et al) . Incidence is increasing partly because of criteria that contribute to its definition as a pathologic entity and due to mammography which has resulted in increased utilization of biopsy. LCIS is multicentric in 60-80% of cases and bilateral in at least 26% of cases. LCIS is ten times more frequent in white women than in African-American women. The age at diagnosis is most commonly between 44-47 years ( vs 52-58 years for DCIS) and 25-35% of women with LCIS develop invasive carcinoma ( 65% of invasive carcinomas that develop are infiltrating ductal, not lobular, carcinomas).
Invasive Carcinomas
Invasive (Infiltrating)Ductal Carcinoma
Invasive ductal carcinoma is the most common cell type, comprising 70% to 80% of all cases. It most commonly affects women in their mid to late 50’s but affects women of all ages.
Structurally it is characrterised by its desmoplastic nature inferring that it has a tendency to incite a fibrous response. The cells form well developed glands and nests, with individual cells being relatively large with large pleomorphic nuclii. The cells also vary in size and shape. The cells in the ducts also undergo necrosis resulting in the dystrophic calcifications, as wll as the accumulation a cheesy material. This is called a comedo carcinoma and the cheesy material can be extruded from the ducts. Multifocality of invasive ductal carcinoma is more frequent when there is a lesion that is is larger than 2-2.5cms.
From a clinical and diagnostic perspective, the lesion is hard to palpation because of its fibrous content. The desmoplastic nature also causes fibrosis of the surrounding connective tissue, resulting in a tugging effect on other structures such as the Cooper’s ligament, the skin or the nipple. Retraction on the ligaments of Cooper presents as peau d’orange – a dimpling of the skin that is reminiscent of the dimpling seen in the skin of an orange. New onset of nipple retraction is also an important warning sign of cancer.
From an imaging perspective, invasive ductal carcinoma is very dense on mammography, and between 70-90% are associated with calcifications, usally shaped as linear casts of the ducts, but are sometimes granular, and usually are more than 5 in number. Calcification is dystrophic in nature, commonly associated with necrosis. Those patients who do not show calcifications pathologically usually show malignancies of lower grade. (low nuclear grade and low grade growth pattern).
An associated ductal carcinoma in-situ is frequently present and comedo necrosis may occur in both invasive areas and areas of intraductal carcinoma.
Invasive ductal carcinoma carries the poorest prognosis among various ductal types. Nuclear and histologic grade have shown to be effective predictors of prognosis. In general the prognosis for the most common histological form which is called invasive ductal carcinoma not otherwise specified (“IDC NOS”) is intermediate. The mucinous, papillary, cribriform, and tubular forms have better survival than the rarer types such as the sarcomatoid and inflammatory carcinomas.
Treatment in general depends on the size, but surgery remains the mainstay of therapy.. In general when the lesion is less than 4cms, the mass is excised and the axillary lymph nodes are sampled. Grading, staging after surgery allows the decision for further adjuvant (post surgical) treatment that may include chemotherapy, radiation therapy, and hormonoal therapy.
When the mass is larger than 4cms modified or full mastectomy is performed, and lymph nodes are sampled, and treatment is tailored to the patients individiual situation.
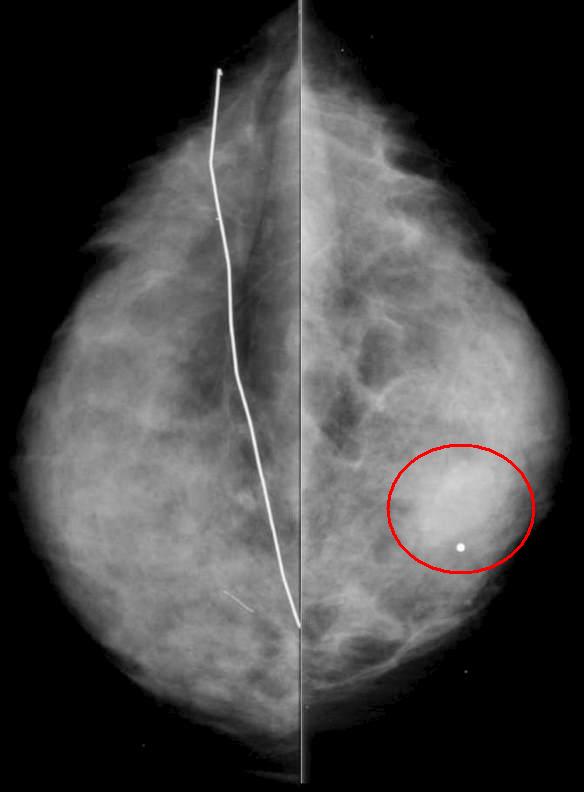 Soft Tissue Mass – Invasive Ductal Carcinoma Soft Tissue Mass – Invasive Ductal Carcinoma |
| The mammogram is from a 45 year old patient with a history of a prior benign biopsy on the left breast now presenting with a hard palpable lump in the right breast. CC view shows subtle asymmetry (red ring). Pathology revealed high grade invasive ductal carcinoma. The patient underwent needle localization and sentinel lymph node dissection followed by radiation therapy and chemotherapy. |
43824r01 45 year old with history of a prior benign biopsy on the left breast now with a palpable lump in the right breast. CC view shows subtle asymmetry. Pathology revealed high grade invasive ductal carcionma. The patient underwent needle localization and sentinel lymph node dissection followed by radiation therapy and chemotherapy. fx asymmetry dx invasive ductal carcinoma breast imaging radiology mammogram mammography Courtesy Priscilla Slanetz MD MPH
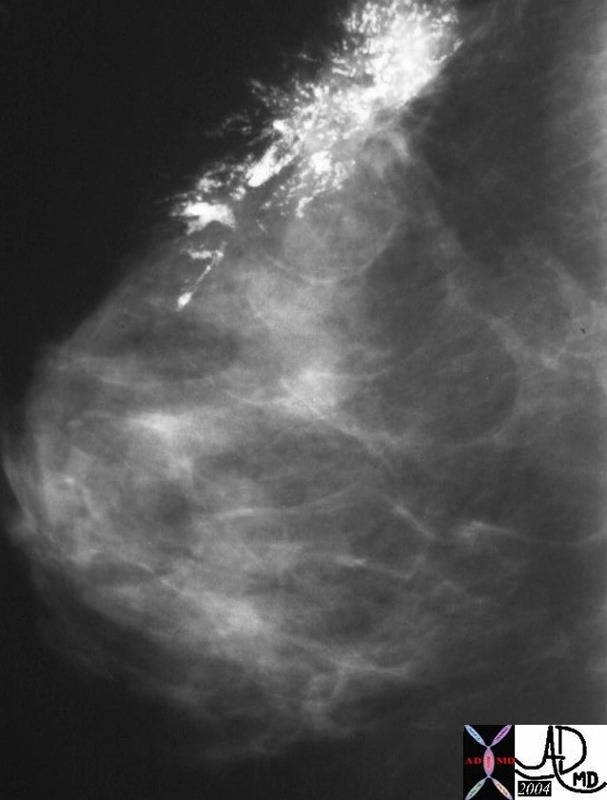 Invasive Ductal Carcinoma DCIS , Positive Nodes Invasive Ductal Carcinoma DCIS , Positive Nodes |
| The mammogram from a 27 year old female who noted a lump in her breast, and shows a left breast a large cluster of pleomorphic calcifications. The patient had a lumpectomy and lymphadenectomy which showed invasive ductal carcinoma, associated with DCIS and positive findings in the lymph nodes.
27975 breast hx 27F left breast lump fx pleomorphic calcification Rx lumpectomy dx intraductal carcinoma with high grade DCIS lymph node positive mammogram mammography Courtesy Priscilla Slanetz MD |
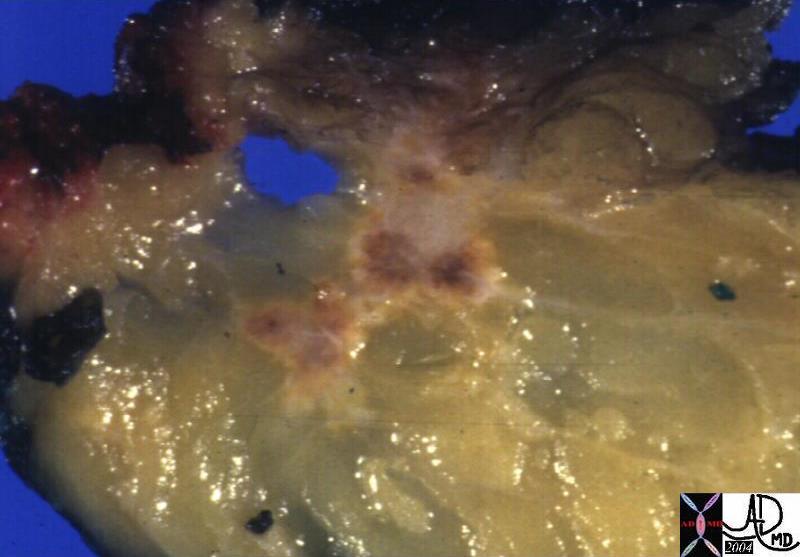 Invasive Scirrhous Ductal Carcinoma Invasive Scirrhous Ductal Carcinoma |
| The grosspathology specimen following surgical resection, was rock hard an gritty to the feel in the region of multiple well defined foci of white to pale yellow lesions with tan colored central foci. Histopathology showed infiltrating ductal carcinoma. The rock hard nature is characteristic of invasive ductal carcinoma, but the gross appearance is non specific.
13553 breast + dx infiltrating ductal carcinoma + grosspathology Courtesy Frank Reale MD DB |
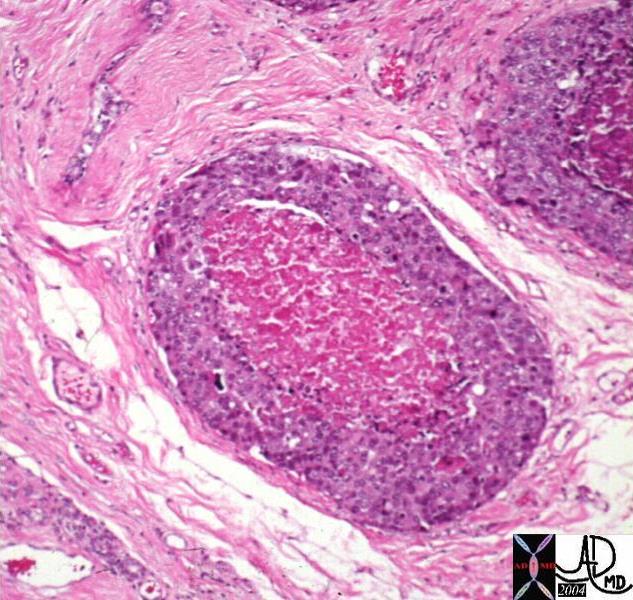 Infiltrating Ductal CArcinoma Infiltrating Ductal CArcinoma |
| The histopathology specimen shows malignant appearing cells within the confines of the duct and a necrotic centre. The nucllii are dark (hyperchromatic) and relatively large compared to the cytoplasm, which are features characteristic of malignancy. However the pathologist indicated that the basement membrane was breached indicating the invasive nature, and inferring a diagnosis of infiltrating ductal carcinoma.
13555 breast + dx infiltrating ductal carcinoma + histopathology Courtesy Frank Reale MD DB |
Invasive Carcinomas
Invasive Lobular Carcinoma
Invasive lobular carcinoma is the less common than invasive ductal carcinoma common cell type.
Infiltrating lobular carcinoma is present in 5%-10% of breast cancers. It is characterized by its bilaterality in up to 20% of patients, and may be multicentric as well
Structurally they are poorly circumscribed, and like the invasive ductal may be associated with some fibrosis, but is not as desmoplastic as invasive ductal carcinoma. It has a typical microscopic appearance characterized by small uniform cells organized in strands with one or two layers of cells organized in a single-file (“Iindian file”). They may encircle the ducts, and often preserve the architecture of the ductsThere is little pleomorphism. It typically grow around ducts and lobules. Lobular carcinomas are frequently multifocal and multicentric and they have a similar prognosis to infiltrating ductal carcinomas. They metastasize to unusal sites such as the meninges, serosal surfaces the GI tract and the retroperitoneum.
Clinically they may present as poorly defined areas of thickening of the breast and these may be hard to detect clinically. Therefore the patients themselves, may not be able to palate these masses on self examionation, and they therefore may be larger than IDC when presenting. (Harminder)
Unlike DCIS, LCIS is not associated with a mass, nor obvious findings on mammography, the diagnosis is usually made under the microscope as an incidental finding on a biopsy specimen.
Mammographically, the lesion is often ill defined, vague, with calcifications only seen in about 2%. (Hilleren). In addition when observed, it usually underestimates the extent of the disease. As a result of the low sensitivity of mammograpphy, ultrsasound and more increasingly MRI (Qayyum)are being used when clinical suspicion is raised, for preoperative planning to define extent, multicentricty and bilaterality. Lobular carcinoma in situ coexists with invasive lobular carcinoma in most cases (70%-80%).
Treatment in general depends on the size, but surgery remains the mainstay of therapy. Breast conserving surgery followed by radiation therapy is recommennded for early stages I or II. Although there is a higher incidenc e of bilaterality, mirror image biopsy, or prophylactic mastectomy is not recommended.
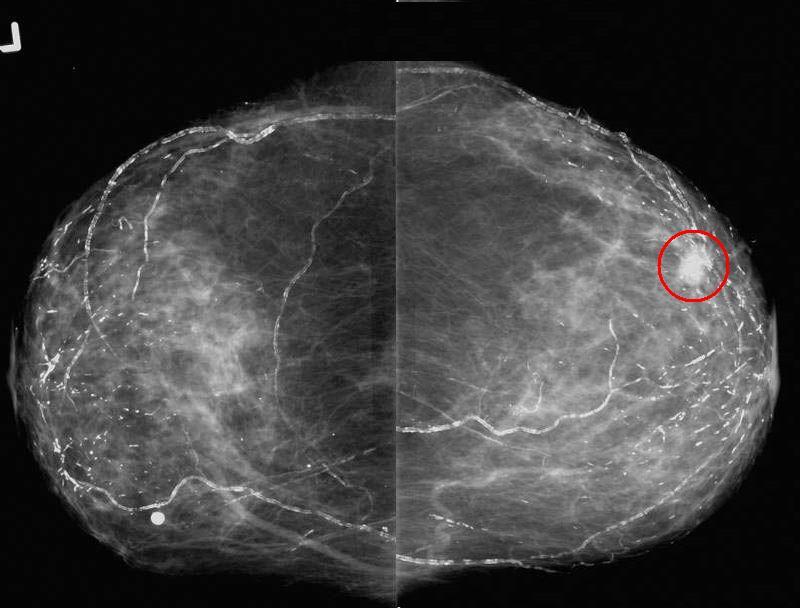 Invasive Lobular Carcinoma Invasive Lobular Carcinoma |
| The patient is an 82 year old presenting for baseline screening mammogram, with a positive family history of breast cancer. Her daughter was diagnosed in her 40’s with breast cancer. The mediolateral oblique ( MLO ) views show an irregular spiculated mass in the upper anterior right breast. Note the vascular and secretory calcifications in both breasts. At pathology invasive lobular carcinoma was diagnosed. Note that in this case the mass was easily identified, by mammography which is not always the case for lobular carcinoma.
breast fx mass fx calcified vascular calcifications ductal calcifications dx lobular carcinoma RX US guided needle loc with surgical excision and XRT breast imaging radiology mammogram mammography
Courtesy Priscilla Slanetz MD MPH 43716r01 |
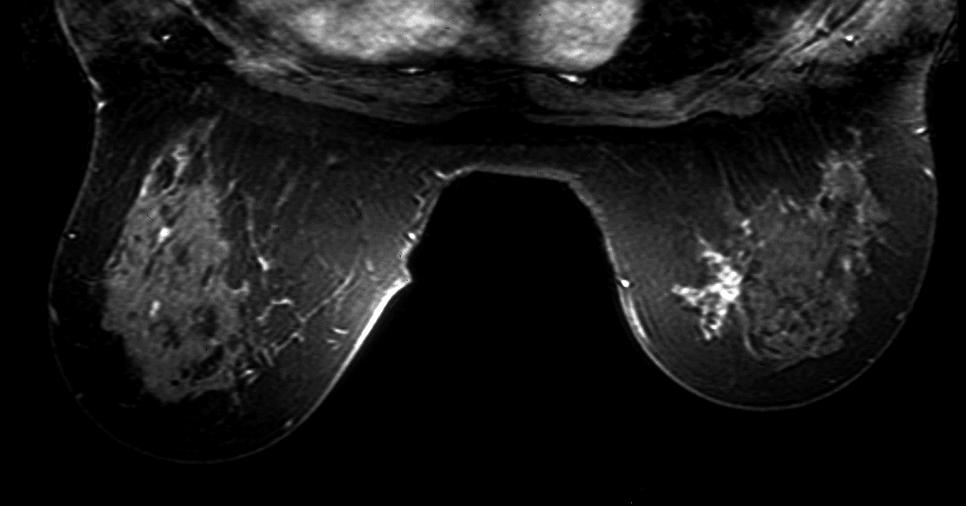 MRI – Invasive Lobular Carcinoma MRI – Invasive Lobular Carcinoma |
| The MRI is from a 54year old female, who had a vague fullness in the left breast. Asymmetric changes showing an enhancing infiltration was shown pathologically to be an invasive lobular carcinoma. MRI is the most sensitive imaging procedure for invasive lobular carcinoma.
42975 Courtesy Priscilla Slanetz MD breast CASE 26: hx 54 year old for screening Dx: Invasive lobular carcinoma MRI DB |
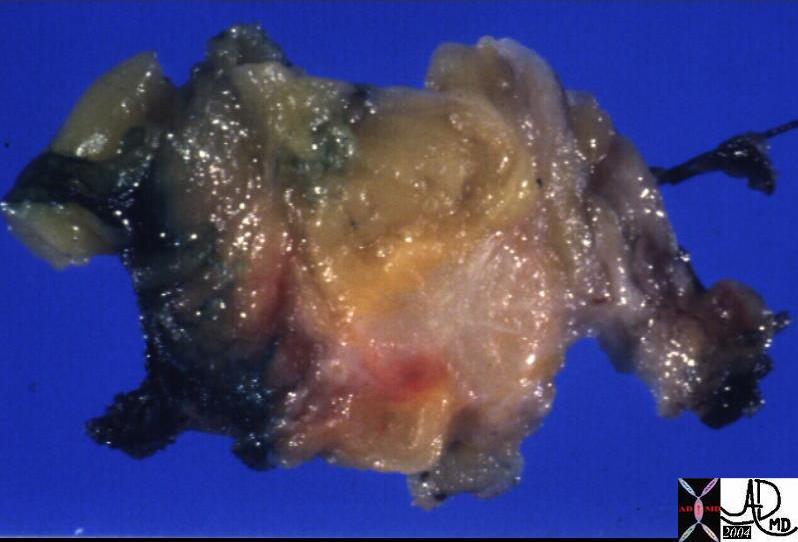 Ill defined Mass in the Surical Specimen Ill defined Mass in the Surical Specimen
Diagnosis Infiltrating Lobular Carcinoima |
| The grosspathology specimen following surgical resection, was ill define with subtle change in texture to the feel. The lesion is the paler tissue in the specimen and is obviously different from the surrounding yellow fat. Histopathology showed infiltrating lobular carcinoma.
13559 breast + fx mass dx infiltrating lobular carcinoma + grosspathology Courtesy Frank Reale MD0 DB |
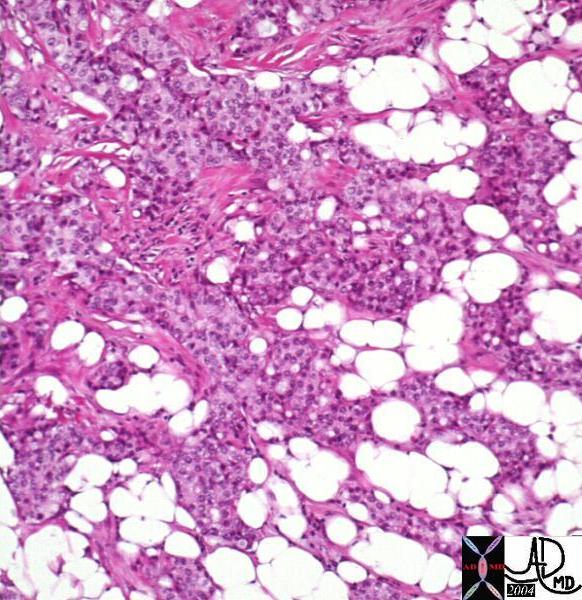 Histopathology of Invasive Lobular Carcinoma Histopathology of Invasive Lobular Carcinoma |
| The histopathology specimen shows features consistent with malignant disease cgharacterized by dark nuclii, and a large nuclear to cytoplasmic nature. All the malignant cells are external to the ducts, beyond the basement membrane making the lesion invasive in nature. The alignment of the cells in columns of cells, in this instance 2-3 layers thick, is characteristic of invasive lobular carcinoma. the formation of cells, sometiimes even only one cell layer thick is called “Indian file”.
13560 breast + fx mass dx infiltrating lobular carcinoma + histopathology Courtesy Frank Reale MD0 DB |
Other Less Common Invasive Carcinomas
Inflammatory Carcinoma
Inflammatory Carcinoma is a malignant condition of the breast, accounting for 1-4% of breast cancer cases, characterized by poorly differentiated ductal carcinoma with tumor invasion into dermal lymphatics, and a universally poor prognosis.
Clinically the breast appears inflammed, red, swollen, and painful, with peau d’orange manifestations in the skin, and nipple retraction. A mass is not always apparent, .
Mammographically the breast is less compressible and dense with skin and trabecular thickening.
Biopsy should include a section of the skin to reveal the presence of the malignancy in the dermal lymphatics in order to make the diagnosis. Despite the name, inflammatory cells are not usually present.
Staging of the tumor is based on the size and extent of metastases.
In general inflammatory breast carcinoma is first treated with chemotherapy before surgery is contemplated, and if surgery is performed, then radiation therapy follows. If the tumor is receptor positive, hormonal therapy is added.
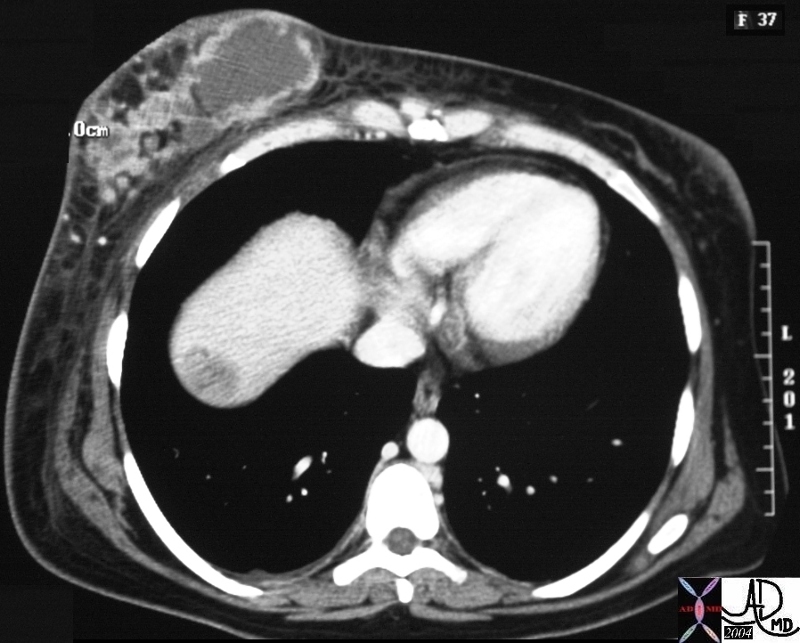 CT scan – Inflammatory Carcinoma CT scan – Inflammatory Carcinoma |
| This CT scan shows a large mass in the right breast, which was pathologically confirmed to be inflammatory breast carcinoma. The superficial skin cannot be fully appreciated but the most medial aspect overlying the breast is thickened. The blood vessels in the lateral aspect of the breast are enlarged, and a liver lesion in the posterior aspect of the right lobe is a known metastasis.
20044 Courtesy Ashley Davidoff MD code breast fx mass inflammatory carcinoma imaging radiology CTscan C+ DB |
Medullary Carcinoma
Medullary carcinoma is a rare cancer of the breast arising from the stromal cells and represents less than 5% or invasive breast cancers. (Meyer) It is characterised structurally by its large size at detection, soft texture, and mobility since it appears to have no significant associated desmoplasia, and has less invasive properties and less commonly metastasizes. The centre of large tumors may be hemorrhagic or necrotic. Pathologically the cells are large,lack glandular morphology and there may be a lymphocytic infiltrate. They all appear be poorly diferentiated, DCIS is usually not seen, and lymphatic and vascular invasion is not seen.
Clinically, medullary carcinoma presents at a slightly younger age (45-55) as a palpable mass, sometimes with a history of explosive growth, commonly in the upper outer quadrant. Rarely, it has been documented in men; it is associated with BRCA-1 mutations in many cases.
It has benign appearing feautures on imaging studies often mimicking benign disease. The mammographic features of medullary carcinoma include the presence of a well defined mass, sometimes lobulated, absent calcifications, often with a complete or incomplete halo. On ultrasound, they are usually large, well defined , oval, round or lobulated, hypoechoic with through transmission. They have similar appearance to fibroadenomas.
Some studies suggest a more favorable prognosis, yet others suggest that the prognosis is the same as other breast malignancies. Overall at 10 years there appears to be no difference in recurrence rates between patients with medullary carcinomas and other subtypes of invasive breast cancer.
| Medullary Carcinoma |
| The histopathological section of the breast shows a medullary carcinoma show cells that are highly pleomorphic with frequent mitotic activity growing in sheets of cohesive cells.
13564 breast medullary carcinoma histopathology Courtesy Frank Reale MD DB |
Other less common tumors include the mucinous or colloid carcinoma which is characterized by its slow growing, soft, well circumscribed nature, that presents as a circumscribed mass in older women. It tends to have a more favorable prognosis.
Tubular carcinomas present in women who are younger (mid 40’s) and are characterized histologically by well formed tubules of malignant cells, often multicentric (10-50%), and sometimes bilateral (10-40%). It has uniformly excellent prognosis.
Paget’s Disease of the Nipple
Paget’s disease of the nippple is a malignant disorder of the nipple areolar complex of the breast, with invasion by cells characterized adenocarcinomatous features accounting for
2-3% of breast carcinomas.. The causative factors are multifactoial, and there is often associated DCIS
Historical notes
The first documentation of Paget’s disease was carried out by John of Ardenne in 1307 who described nipple ulceration in a male priest that evolved into breast cancer. In 1874 Sir James Paget documented the association of the ulceration of the nipple with an underlying breast cancer in 15 patients, but erroneously speculated that the skin condition was benign. Such a conclusion was then rectified in 1881 by Thin who concluded that the skin condition was a malignancy.
Pathogenesis
There are two main theories for the origin of Paget’s disease of the nipple: the epidermotropic theory ( Paget cells arise in breast ducts and migrate to the epidermis) and the intraepidermal transformation theory ( Paget cells arise from the terminal portion of the lactiferous duct). The first theory is supported by the fact that more than 97% of patients with Paget’s disease of the nipple have an underlying breast carcinoma.
Clinical presentation
Paget’s disease typically presents as erythema and scaling of the nipple skin that progresses to crusting, skin erosion and ulceration if left untreated. Symptoms may include pruritus, tingling, hypersensitivity, burning or pain. Many diseases must be included when differentiating Paget’s disease from other conditions that lead to erythema and scaling of the skin. The differential diagnosis therefore includes: eczema, contact dermatitis, postradiation dermatitis, and Paget’s disease. Bilateral changes are most consistent with eczema and contact dermatitis. Less common diseases in the differential diagnoses include nipple adenoma and a variety of skin cancers ( melanoma, Merkel cell carcinoma, basal cell carcinoma, squamous cell carcinoma).
The diagnosis is obtained in a variety of ways including scrape cytology, shave biopsy, 2mm punch biopsy, wedge incisional biopsy or nipple excision. Immunohistologic staining is useful in the differentiation of Paget’s ( + for keratins- CK-7, CAM-5.2, + for S100) from other malignancies.
Conventional imaging is usually unable to detect the underlying neoplasia’ though recently the utility of MRI to distinguish between supeerficial disease and deep disease has been utilized.. (Echevarria).
The typical histologic appearance of the Paget cell is that of a large pale-staining cell with round or oval nuclei and prominent nucleoli . Paget cells are between the normal keratinocytes of the nipple epidermis ( single cells in superficial layers and clusters near the basement membrane).
Since Paget’s disease presents earlier it tends to have a more favorable prognosis. However prognosis depends on the presence of a breast mass. When present the prognosis worsens, with a survival rate of 40% at 5 years and about 30% at 10 years. In the absence of a mass survival at 5 years is 90-94%, and at 10 years it is 82-90%
Incidence
Paget’s disease is diagnosed pathologically more frequently than it is clinically . Its clinical incidence ranges from 0.5% to 2.8% (mean 1.3%) whereas its pathological incidence is approximately 4%.
Treatment
Treatment of Paget’s disease varies depending on the presence of underlying breast carcinoma or DCIS. Mammogrephic evaluation looking for evidence of breast cancer is therefore part of the routine evaluation of patients with Paget’s disease (bilateral mammograms). If available, preoperative MRI of the breast can identify occult disease. When possible breast conserving surgical intervention should be considered. THe combination of radiation to breast conserving surgery has been shown to decrease recurrence rates. For patients undergoing mastectomy or breast preservation, the decision of evaluation and treatment of axillary nodes should be reserved for invasive breast cancers.
Cystosarcoma Phyllodes (Phyllodes Tumor)
Phylloides is a rare type of breast disease, more commonly benign, but may be malignant, (10-15%) usually presenting in women with an average age of 70, structurally characterised by features of stromal ori rather than epithelial origin and histolgy that is reminiscent of a fibroadenoma., but with a cellular stroma and epithelial lined branching clefts. The benign disease can be locally recurrent afrter resecction which is another characteristic feature of the disease. Its name originates from the Greek words “sarcoma” meaning fleshy, and “phyllo” meaning a leaf, but because it is more commonly a benign tumor, the term Phyllodes tumor is favored over the more malignant sounding name of “sarcoma”
Clinically it presents as a large, well circumscribed, rapidly enlarging mass in a an elderly patient.
On mammography it is a well circumscribed, solid mass, usually in the 5cms range, and resembles a fibroadenoma. However fibroadenoma presents in young premenopausal patients, and the phylloides tumor is seen in post menopausal elderly patients. On ultrasound the tumor is seen as well circumscribed, and may have cystic spaces in the matrix.
The treatment includes wide local excision with a margin of at least 2cms on lesions <5cms, and a 5cms margin on lesions >5cms. Nodal resection is reserved for those patients where nodes are large enough to raise clinical suspicion. Chemotherapy and radiation therapy are not used in the treatment of phyllodes tumors. Hormonal intervention also has no role.
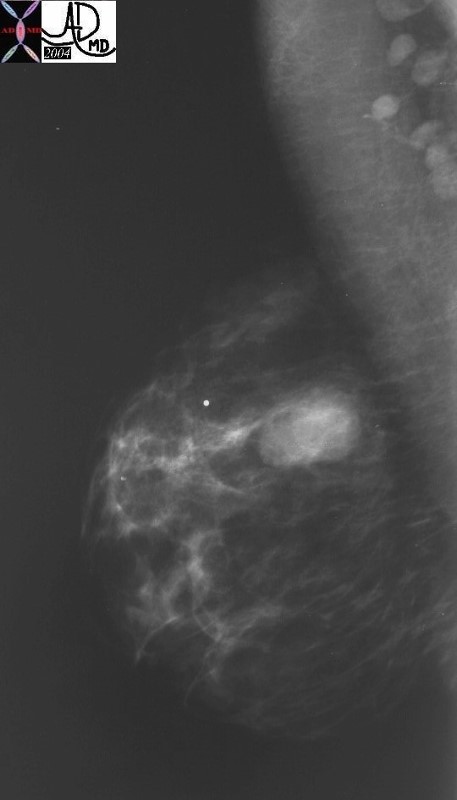 Well Circumscribed Mass – Phyllodes Tumor Well Circumscribed Mass – Phyllodes Tumor |
| The mammogram is from a 36 year old female who presented with a palpable mass. There is an obvious well circumscribed mass with round borders. In a patient od tgis age fibroadenoma would have been the most likely diagnosis. However the pathology showed cystosarcoma phyllodes.
28249 breast hx 36F fx mass dx cystosarcoma phylloides mammogram mammography Courtesy Priscilla Slanetz MDDB |
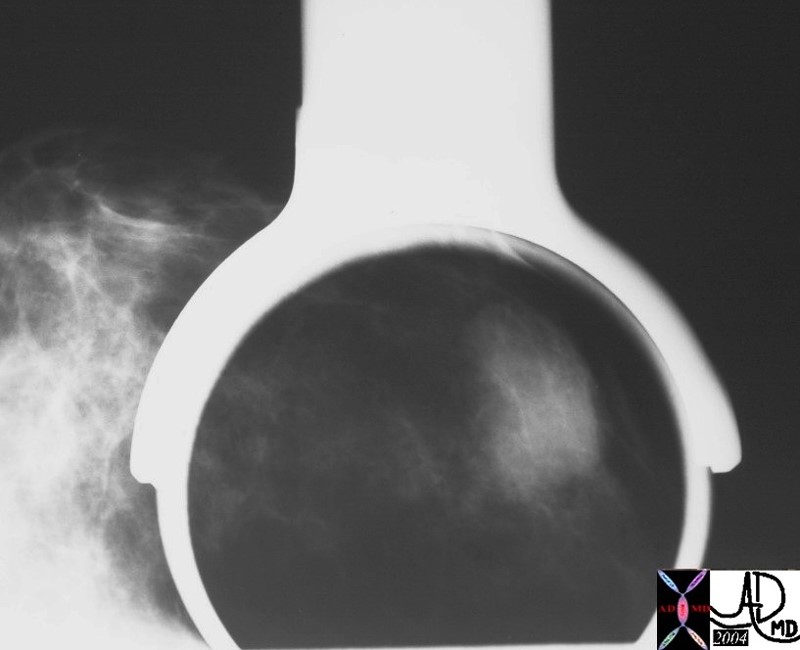 Magnified View of the Well Circumscribed Phyllodes Tumor Magnified View of the Well Circumscribed Phyllodes Tumor |
| 28251 breast hx 36F fx mass dx cystosarcoma phylloides mammogram mammography Courtesy Priscilla Slanetz MD DB |
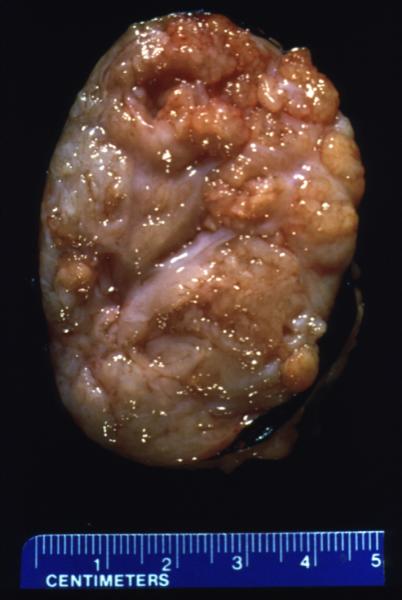
Gross Pathology of the Phyllodes Tumor |
| The gross pathology specimen shows a 5cms, well circumscribe mass with white fibrous bands and a lobular pattern. Histopathology was consistent with Phyllodes tumor.
11090 breast mass cystosarcoma phylloides 5 cms grosspathology Courtesy Frank Reale MD |
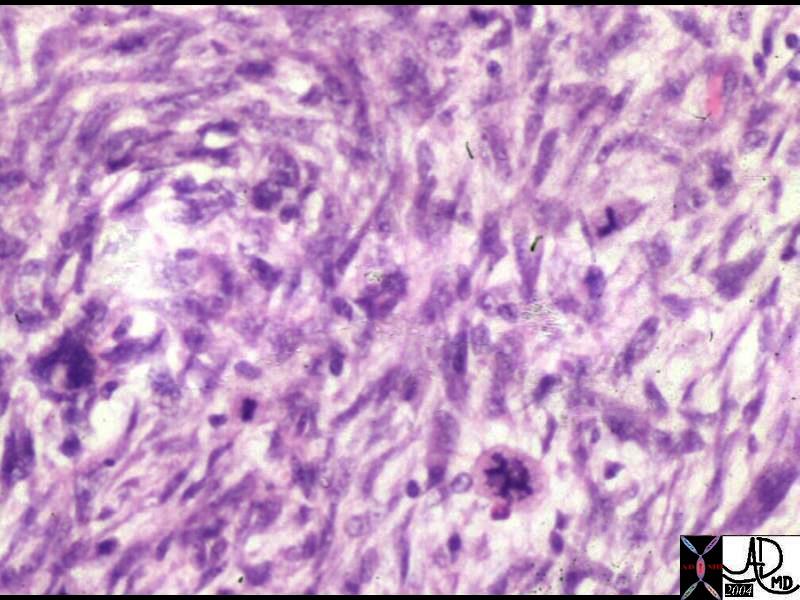 Histopathology of the Phyllodes Tumor Histopathology of the Phyllodes Tumor |
| The histological section shows findings characteristic of the Phyllodes tumor. The cells have a stromal and fusiform shape that resemble fibroblasts. In this section there is a high degree of cellular atypia.
13537 breast dx cystosarcoma phylloides carcinoma histopathology Courtesy Frank Reale MD DB |
Summary of the Pathological Characteristics of breast Carcinoma
| Intraductal carcinoma of the breast (DCIS) |
Tumor cells fill ducts, tumor cell necrosis results in cheese-like consistency
|
| Invasive ductal carcinoma |
Most common type, characterized by tumor cells arranged in cords, islands, and glands embedded in a dense fibrous stroma; abundant fibrous tissue results in firm consistency |
| Paget disease of breast |
Eczematoid lesion of the nipple or areola; neoplastic Paget cells, characteristic large cells surrounded by a clear halo-like area, invade the epidermis; underlying ductal carcinoma almost always present |
| Lobular carcinoma in situ (LCIS)
|
Clusters of neoplastic cells fill intralobular ductules and acini; may lead to invasive carcinoma many years later (in the same or contra lateral breast); often bilateral at the time of diagnosis
|
| Invasive Lobular Carcinoma
|
One or two layers of cells organized in a single-file (“Iindian file”). May encircle the ducts, and often preserve the architecture of the ducts. Grow around ducts and lobules. Lobular carcinomas are frequently multifocal and multicentric |
| Medullary carcinoma |
Cellular with scant stroma; soft, fleshy consistency; characteristic lymphocytic infiltrate, prognosis better than that for invasive ductal carcinoma
|
| Mucinous carcinoma |
Pools of extracellular mucus surrounding clusters of tumor cells; gelatinous consistency; prognosis better than for invasive ductal carcinoma
|
| Inflammatory carcinomas |
Lymphatic involvement of skin by underlying carcinoma, causing red, swollen, hot skin resembling an inflammatory process; peau d’orange, poor prognosis |
Clinical Approach
The approach to the patient with suspected breast cancer should always follow solid clinical principles that include a directed history, and thorough directed clinical examination. These are followed by investigation with imaging, and documentation by biopsy. Once the diagnosis is established, grading and staging of the disease is necessary with the initial goal of establishing whether surgery is indicated.
History Related to the Primary Site
The most common symptom associated with breast cancer is a lump or alteration in the shape of breast. The breast cancer by itself may be painless unless there is a superimposed infection or bony lesion. There may be a change in the nipple in the form of nipple retraction, or nipple discharge. The inflammatory breast cancer may present with pain and redness.
Questions in the clinical history that are relevant include how long the patient has noted the mass, how big the mass is, where it is located, and whether it feels hard or soft. Its relationship to the menstrual cycle, and the presence of associated symptoms such as pain, nipple retraction, nipple discharge, itching, or skin changeare relevant questions.
Directed history probing the possibility of metastatic disease includes symptoms of back pain, bone pain, breathing dificulties, local neurological symptoms, or jaundice. Weight loss, malaise, weakness and fever reflect systemic manifestations of cancer.
The overall risk of the patient is probed by finding out the genetic links of the patient. The incidence is 4 times higher in patients who have a mother or sister with the disease. The risk is also much higher in patients who have family members with a history of both breast and ovarian cancer. The risk is higher in Jewish Ashkenazi women, but lower in African American, Japanese, and Taiwanese women. The patients who have ataxia telangiectasia have a 4 times increased risk. Inherited breast cancer though, only makes up a quarter of all breast cancer cases.
Other diseases that have increased risk include patients with a previous history of breast disease that include breast carcinoma, DCIS. LCIS, complex fibroadenoma, radial scar, sclerosing adenosis, ovarian carcinoma, or endometrial carcinoma.
It is important to ask questions that relate to both lifetime exposure to estrogen, as well as hormonal therapy that incorporates estrogen. The onset of menarche and menopause, obstetric history, and duration of breastfeeding have relevance to the disease. Directed questioning on the use of estrogen containing oral contraception, and hormone replacement therapy both raise the risk.
Signs
The exam for a patient who has suspected breast cancer should include an exam of both breasts.The directed clinical exam explores the size of the mass, its texture, mobilty, and symmetry. Evaluation of the nipple, and overlying skin looking for tethering, inversion, and peau d’orange follows. Nodal assessment in the subpectoral region, axilla, and supraclavicuar area is the next step.
Systemic evaluation for metastases should include evaluation of the sclera for jaundice, neurological exam for focal motor or sensory deficit, the lungs for pleural effusion, and the spine for focal back pain.
Investigations.
Lab Tests
Lab tests are not very helpful in breast cancer. A high calcium level may be seen in bony metastatic disease. Low blood count may be seen due to bone marrow involvement
Imaging
Imaging forms the epicenter of breast cancer management.
Imaging Strategies
All women are recommended to undergo breast cancer screening in form of annual mammogram once they are above 40 yrs of age. After treatment for breast cancer women should continue to undergo annual mammogram except for the women who have undergone bilateral mastectomies. The most important imaging is mammogram however ultrasound may be requested in women with dense breasts or cystic lesions. The MRI may be recommended in women with high risk features such as history of breast cancer in the past , family history or presence of mutations that confers high risk of breast cancer.
Mammography:
Aim:
Mammography is an x-ray technique designed to detect cancers at an earlier stage than physical examination. Earlier detection translates into earlier stage cancer, and hence, improved long-term prognosis. Most cancers detected by mammography are non-palpable and are ductal carcinoma in situ, which is highly curable.
Indications: Current screening guidelines are based on a patient’s individual risk. For the average woman, annual mammography starting at age 40 years is recommended. If the patient is high-risk (i.e. has a first degree relative diagnosed with breast cancer), she should begin screening a decade prior to the relative’s age at diagnosis or 40 years, whichever is soonest. Women who are known carriers of the BRAC-1/BRCA-2 mutations often begin screening in their 30’s as they have an 80% lifetime risk of developing breast cancer (10-12% lifetime risk for average patients). Nearly 40% of women treated with mantle radiation for Hodgkin’s disease prior to the age of 25 years will develop breast cancer by age 45, so these women begin annual screening mammography in their 30’s as well.
Diagnostic mammograms are indicated if a patient has a specific problem, such as pain, lump, thickening, or nipple discharge. This examination is performed on symptomatic patients over the age of 30 years and is usually combined with an ultrasound targeted to the area of concern.
Contraindications: The benefits of any imaging test should always outweigh the risks. In general, there are no true contraindications to mammography. However, mammography is not performed on women who are in the first trimester of pregnancy (unless there is substantial clinical suspicion for breast cancer) or who have an active mastitis. As patients must be able to sit or stand upright, bedridden patients can not undergo the examination.
Advantages: In multiple randomized screening studies, mammography has been shown to reduce breast cancer mortality by up to 30%. This mortality reduction even applies to women in their 40’s, and more recent studies suggest that these women may benefit even more with reductions up to 45%.
Disadvantages: For the rare patient, the breast compression associated with mammography is extremely painful and can not be tolerated. If this is the case, the patient is asked to take motrin or ibuprofen an hour prior to her appointment and to schedule the study during the first week of the menstrual cycle (if applicable).
Method
Patient Preparation: Patients are requested not to wear antiperspirants or powders on the day of the study as the metals in these compounds can appear similar to microcalcifications. Deodorants are usually do not interfere with the images. Patients are also asked to bring all of their prior outside mammograms with them to their appointment as the radiologist will need these as they interpret the current study.
Technique: The patient is asked to undress from the waist up and put on a gown. The technologist takes the patient into a mammography room and reviews the patient information sheet for accuracy and completeness. Each breast is then positioned for the craniocaudad (CC) and mediolateral oblique (MLO) views. The patient waits until the technologist determines that the images are of good quality. She then can leave the center. The radiologist will interpret the screening study later that day. If the patient has a symptom, she will wait at the center until the radiologist reviews the films, as there may be a need for additional mammographic views or possibly even an ultrasound.
Results: Recall for additional imaging from a screening examination ranges from 3-12% depending upon the center. The positive predictive value of biopsy for most centers ranges between 20-40%.
The false negative rate of mammography is approximately 5-15%, depending upon the experience of the interpreting radiologist. Double reading (i.e. two radiologists interpret every screening study) and/or the use of computer-aided detection (CAD) can help reduce the false negative rates by up to 40-60%.
Conclusions: Mammography is a safe, effective tool for the early detection of breast cancer and reduces mortality from breast cancer by up to 30%.
Ultrasound of the Breast
Aim:
Ultrasound uses sound waves to image the breast. This modality is predominantly designed to differentiate a cystic mass from a solid mass. In addition, ulrasound can be used to guide percutaneous procedures, such as fine needle aspiration, core needle biopsy, or percutaneous excision.
Indications:
Ultrasound is indicated to: 1) Evaluate a palpable abnormality on physical exam (focal pain, lump, thickening); 2) Evaluate a mammographic finding (focal mammographic lesion); 3) Evaluate focal pain persistent for greater than 3 months; or 4) To guide a percutaneous procedure such as a core biopsy or fine needle aspiration (FNA). Although there are a few studies using ultrasound to screen for breast cancer, this is not widely embraced as the modality is highly operator dependent. In addition, there are no large randomized studies showing that screening ultrasound reduces mortality from breast cancer.
Contraindications:
There are no known contraindications to breast ultrasound.
Advantages:
Ultrasound is a safe technology with no known side effects. It does not require breast compression. Although usually performed with the patient supine or supine oblique, it can be performed in the upright position in wheelchair bound patients. It offers a means to better characterize palpable and mammographic findings and provides a mechanism to biopsy lesions percutaneously.
Disadvantages:
Ultrasound is highly operator dependent. Despite standard planes to image abnormalities, the detection of an abnormality depends on the skill of the operator. Although there are features on ultrasound which favor benign disease from malignancy, ultrasound can not reliably differentiate solid lesions, and therefore, biopsy is often necessary.
Method
Patient Preparation:
There is no particular preparation for this examination, except that we do ask patients to bring all previous breast imaging studies with them to their appointment.
Technique:
Usually, the patient is asked to lie down on a stretcher. With their arm above their head and in a supine or supine oblique position, the technologist or radiologist places gel on the skin and scans the patient with a linear 12-17MHz transducer in the radial and antiradial planes. Images are usually acquired in both planes if an abnormality is identified and only in one plane if the study is normal. If there is a palpable abnormality, the patient is asked to put their finger on the spot prior to scanning.
Results:
Ultrasound has high specificity for the diagnosis of simple cysts.
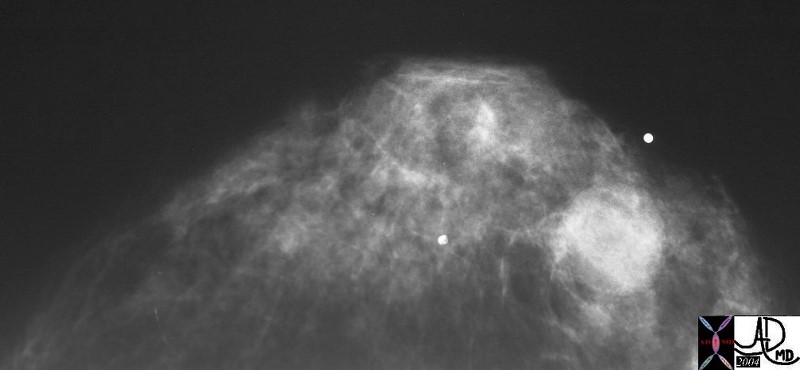 Well Defined Mass Well Defined Mass |
| The mammogram shows a well defined mass in the breast, which cannot be further characterized. It could be cystic ,in which case no further workup would be necessary, or it could be solid. Ultrasound is extremely helpful in this situation.
mmamogram28239 breast fx mass fx well circumscribed dx simple cyst mammogram mammography Courtesy Priscilla Slanetz MD DB |
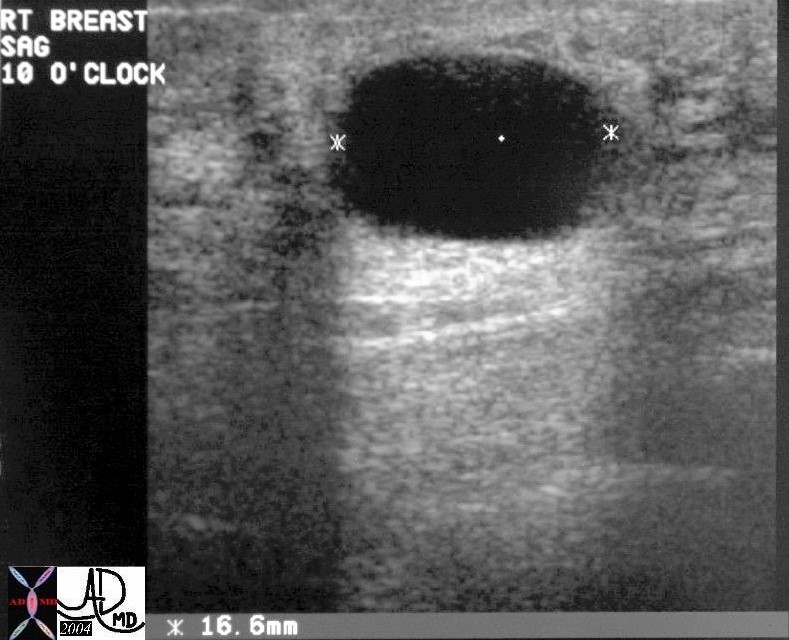 Ultrasound Showing a Cyst Ultrasound Showing a Cyst |
| The ultraound shows a well defined mass in the breast, which is anechoic with through transmission visible as a column of white behind the black cyst. Since this is a simple cyst no further workup is necessary.
28241 breast fx anechoic through transmiision dx cyst USscan Courtesy Priscilla Slanetz MD DB |
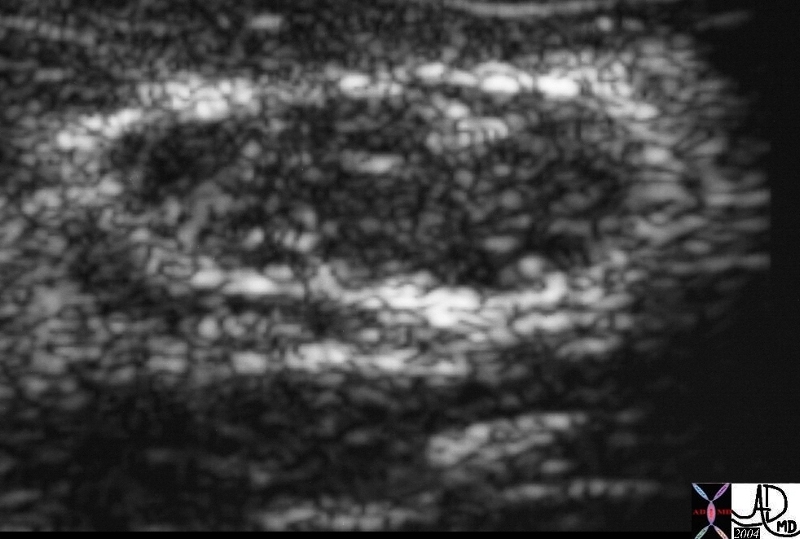 Ultrasound Showing a Solid Lesion – Fibroadenoma Ultrasound Showing a Solid Lesion – Fibroadenoma |
| The ultraound in this instance shows a solid lesion, meaning that there are echoes within the lesion. Biopsy is therefore necessary. This case represented a fibroadenoma.
This a an Us of a solid lesion of the breast. In his case the lesion was a fibroadenoma22260 Courtesy Ashley Davidoff MD code breast fx mass fx solid imaging radiology USscan DB
28241 breast fx anechoic through transmiision dx cyst USscan Courtesy Priscilla Slanetz MD DB |
Ultrasound Showing a Solid Lesion – Invasive Ductal Carcinoma |
| The ultraound in this instance shows a hypoechoic lesion, with irregular ill defined borders and no through transmission, and in fact shadowing or blackness is behind the lesion. This is not a cyst and biopsy is therefore necessary. This case represented an invasive ductal carcinoma.
42933 Courtesy Priscilla Slanetz MD breast Case 16 51 yo screening fx mass Dx: invasive ductal carcinoma USscan Carcinoma typically ill-defined, irregular, spiculated or microlobulated hypoechoic acoustic shadowing taller than wide (transverse to AP ratio < 1.4) Ultrasonographic Analysis of Breast Masses DB |
Conclusions:
Ultrasound is a safe modality to evaluate a palpable abnormality or a suspicious mammographic finding. It can reliably differentiate cysts from solid masses.
MRI of the Breast
Aim:
Magnetic resonance imaging (MR) of the breast uses a high-field magnet to image the breast. Multiplanar imaging with different sequences prior to and following contrast administration permits evaluation of the breast tissue. Interpretation of MR studies entails a combination of lesion morphology and enhancement pattern. Lesions that demonstrate early rapid uptake of contrast with a plateau or delayed washout over time are most suspicious for malignancy and warrant biopsy. Gradual uptake of the contrast over time favors a benign process.
Indications:
Current indications for this study are as follows: 1) To determine the integrity of silicone breast implants; 2) To determine disease extent in a newly diagnosed breast cancer patient, particularly younger patients with dense tissue; 3) To identify the breast primary in patients with positive axillary lymph nodes and negative physical exams; 4) To differentiate scar from recurrence in selected patients previously treated for breast cancer; 5) To further characterize an incompletely evaluated suspicious mammographic finding; 6) To monitor response to neoadjuvant chemotherapy in patients with locally advanced breast cancer; and 7) To screen high-risk women, particularly BRCA-1 and BRCA-2 known carriers.
Contraindications:
The standard contraindications to MR imaging apply. In addition, as intravenous gadolinium is given for almost all studies, an allergy to gadolinium would preclude this examination. Relative contraindications include pregnant women as the safety of gadolinium to the fetus is unknown.
Advantages:
MR imaging of the breast is a highly sensitive test with sensitivities approaching 100% for the detection of invasive carcinoma.
Disadvantages:
The specificity is variable depending upon the specific sequences employed and the experience of the interpreting radiologist. In addition, the imaging sequences are not standardized across sites in the U.S..
Method
Patient Preparation:
Patients must be screened for contraindications to MR imaging. Premenopausal patients and cycling postmenopausal patients should be imaged between days 5-15 of the menstrual cycle in order to control for hormonal fluctuations.
Technique:
The patient lies prone on the scanner with her breasts pendent and in mild compression in the dedicated breast coil. An intravenous line is placed through which contrast will be administered. Every center utilizes different sequences for imaging the breasts. In general, most centers acquire images in the sagittal and/or axial plane prior to and following contrast administration. During the contrast injection, dynamic imaging is performed such that images of the entire breast volume are acquired every 1-2 minutes for up to 8-10 minutes following the start of the injection. Using CAD software (Confirma or DynaCad), the enhancement curves can be generated. The entire study usually takes approximately 30 minutes to perform. The radiologist then interprets the images by looking at the lesion morphology and its enhancement pattern.
Results:
MR imaging of the breasts is a highly sensitive test with sensitivities approaching 100% for the detection of invasive carcinoma. Its sensitivity for ductal carcinoma in situ ranges between 10-90% depending upon the center and its sequences. Specificity for this study ranges between 75-90%, once again depending on the specific sequences employed. The false negative rate of MR is unknown.
Conclusions:
MR imaging of the breast serves as a useful adjunct to other breast imaging modalities and for screening high-risk women, particularly known carriers of BRCA-1 and BRCA-2. MR-guided percutaneous core needle biopsy is now available at most centers permitting minmally invasive diagnosis of suspiciously enhancing lesions detected only on MR imaging.
PET Scan
PET scan is not usually recommended for the work up of breast carcinoma. It is limited by the size of the tumor and tumors less than 1cms may not be identified. While it is sensitive and able to detect invasive ductal carcinoma, it is not sensitive to in situ carcinomas and less sensitive to lobular carcinoma.
Reporting the Findings on Imaging Studies
Breast Imaging Reporting and Data System (BI-RADS)
BI-RADS is a standardized method of reporting mammography studies allowing for a quality assurance mechanism, consistency and clarity of the reports. It was developed by the American College of Radiology as a standard for rating the liklihood of cancer by setting up levels of suspicion. BI-RADS system takes into account presence of a discrete nodule, calcifications, and architectural distortion of focal asymmetric densities.The radiologists scores the findings from 1-6 providing the referring physician a standardized report on the liklihood of cancer in their patient.
BI-RADS Scores
| Category |
Assessment |
Criteria |
| 0 |
Incomplete |
The mammogram or ultrasound does not provide enough information to make a clear diagnosis; follow-up imaging is necessary |
| 1 |
Negative |
routine screening recommended |
| 2 |
Benign |
A definite benign findingis present; routine screening recommended |
| 3 |
Probably Benign |
Findings that have a high liklihhood of being benign six-month short interval follow-up, then every 6-12 months for 1-2 years |
| 4 |
Suspicious Abnormality |
Not characteristic of breast cancer,
biopsy should be considered |
| 5 |
Highly Suspicious of Malignancy |
Lesion that has a high probability of being malignant
biopsy needed |
| 6 |
Known Biopsy Proven Malignancy |
Lesions known to be malignant that are being imaged prior to definitive treatment; assure that treatment is completed |
Diagnosis
Once an abnormality is detected in the breast by palpation or imaging studies, the gold standard for diagnosis is hitospathological exam of a biopsy specimen. The optimal method to do biopsy is decided upon size, depth and nature of abnormality. Cystic lesion may be aspirated under ultrasound guidance. Fine needle aspiration may be done which may help in diagnosis. Core biopsy is an excellent method, the lesion may be studies in greater detail as well as staining is possible for hormone receptors. It may be done by stereotactic guidance or needle localization. Rarely when a lesion is not accessible incisonal biopsy may be needed.
Biopsy
Histopathological examination of breast biopsy of the suspicious lesions remains the gold standard of diagnosis. This is most commonly performed using imaging guidance. In the event that usual techniques are indeterminate or fail then surgical biopsy is performed.
The image guided techniques include ultrasound, stereotactic (X-ray using specially designed mammography equipment), and MRI.
Ultrasound is the most commonly performed because it allows real time visualization of the needle and the lesion, and avoids radiation. Stereotactic guidance is used when calcifications are present since it is more sensitive than ultrasound to identify this important aspect of the lesion. MRI is performed when the lesion cannot be seen by ultrasound or mammography. Surgery is performed when the above techniques fail.
Percutaneous breast biopsy is usually performed as an outpatient using imaging guidance, commonly by ultrasound, but mammography and MRI are also utilized.
Aim
The aim of the procedure is to obtain sufficient tissue to diagnose malignancy and to characterize the tissue relating to its specific histopathological type, grade and hormonal receptor status.
Indications
Based on the BI-RADS system the lesions for biopsy are most commonly those designated as BI-RADS score 4 or 5. There will be some uncommon situations where a biopsy for a category 3 lesion is indicated, (eg inability to obtain mammographic follow up, anxiety about the lesion, planned pregnancy, planned breast cosmetic surgery) but the goal is to avoid biopsy of benign disease.
Since many lesions identified by mammography are impalpable, and are seen only by mammography or ultrasound, image guided biopsy is utilized. Ultrasound guided procedures are most widely used since they are real time inferring that the needle can be observed as it is advanced into the lesion. Ultrasound also avoids radiation, and is relatively inexpensive. However when microcalcifications are present, tor when the lesion cannot be seen by ultrasound then stereotactic biopsy is employed. MRI guided procedures are used when the the lesion can only be seen by MRI.
Contraindications
There are no absolute contraindications and only relative contraindications. Obviously if the lesion cannot be definitely seen it cannot be biopsied. In patients who cannot come off anticoagulation, there is a relative contraindication to the procedure, but this circumstance is rarely encountered.
Advantages
The risks of the procedure are low, and the need to diagnose the disease, evaluate the type, and grade the tumor are essential to treatment planning.
Disadvantages
There are no disadvantages other than the innate concerns of radiation in the case of stereotactic method, and the risk of bleeding, infection and low seeding the tumor.
Method
Patient Preparation
The procedure, the technique, and the risks and benefits need to be explained to the patient in a quiet and confident manner and informed consent is necessary.
Aspirin prophylaxis should be stopped a week before the procedure, and any other anticoagulation should be discontinued to enable the INR to be below 1.5. The patient does not have to be NPO
Equipment
There are a variety of needles and cutting devices that are used. Cytological apsirates are no longer used by radiologists, who only use only core technique. The cutting needles which are used for obtaining a core are usually 14 gauge. More recently use of the vacuum assisted devices (VAD) are being utilized , since they are able to obtain larger samples of tissue. The 9 French cutting needleincreases the yield of invasive carcinoma in a DCIS environment, because the larger sample size. In general these are used for the stereoscopic and MRI guided procedures.
Technique
All of the procedures require sterile technique, and local anesthesia.
Whichever technique is used the principles include;
find the shortest distance to the lesion and transgress the least amount of tissue
biopsy it,
mark the biopsy site with a clip,
prove that the appropriate tissue was acquired by specimen X-ray for microcalcifications, or post procedure mammogram to prove that the lesion has disappeared.
Fine needle aspirate
Fine needle (FNA) using a 21 gauge needle is the least invasive and most commonly used needle and has a sensitivity of 50-95%% and a specificity of 95-100%. As stated radiologists do not use this technique due to the limited amount of information that it provides.
A slow back and forth motion in the lesion observing the needle in real time, until some material is seen in the bottom of the barrel of the syringe.
The aspirate is transferred onto glass slides, and is spread by a second glass slide in order to ensure that the material thinly distributed. This is best done by an attending cytopathology technologist. Staining techniques are individualized by the preference of specific pathology laboratories.
Core Biopsy
Core biopsy specimens may be obtained in a variety of ways. A 14 gauge trucut needle using a spring loaded firing device, facilitates needle penetration of hard fibrous elements in the tumor which may otherwise be difficult to penetrate. The volume of tissue is larger than the aspirate, and the architecture of the tumor can be appreciated. Once the specimen is obtained the material is transferred to formalin. If the lesion was known to contain calcifications then specimen radiographs are obtained to ensure accuracy of sampling.
A pressure dressing is usually applied for 24 hours after the procedure to prevent or limit bleeding.
Vaccuum Assisted Device
The VAD is usually an 11 gauge percutaneous system that is vaccum driven used in stereotactic biopsies and in MRI. It is particulalrly helpful in the DCIS environment where a small pocket of invasive carcinoma may be present. In this instance the larger the sample the more likely one is able to identify invasive disease.
On occasion it is used to excision benign lesions that are less than 15mm in size. The purpose is to avoid surgery for benign disease.
Results
The fine needle procedures appear to be adequate if the only question is the presence or absence of malignancy. It requires cytopathologists, who are experienced in the technique. It proves to be inadequate for treatment planning since detailed information of the architecture grade, and histologic type is needed, that can only be provided by core specimens. The Tru Cut needle which is a well known core biopsy needle has a sensitivity that ranges between 64%-84%, and the 14gauge Biopty cut has a sensitivity of 88-98%. Both have specificity of 100%.
Complications
The complications of the procedure include bleeding and infection. Hematomas have become more frequent as the sizes of the needles have increased in size. The MRI guided procedures are particulalrly prone to hematoma because while the patient is in the scanner the ability to identify bleeding or to compress bleeding if it is present is restricted, since the patient is out of reach and observation while in the gantry.
Conclusion
Biopsy of an equivocal finding in the breast is an essential and key step in the diagnosis and management of breast carcinoma. The histopathological type, grade, and hormone receptor status are necessary before embarking on a treatment program. Ultrasound and core biopsy technique are favored in most cases, while stereotactic biopsy is favored when microcalcifications are present, or the lesion cannot be seen by ultrasound. MRI guided procedures are utilized when it is the only modality able to visualize suspicious areas. Needles have become larger so that current sized needles range from 9 gauge to 14gauge, though some institutions use 18 gauge for the cores and 21 gauge for cytology. Surgical excisional biopsy is used for lesions that reveal equivocal pathological findings such as atypical intraductal hyperplasia, or in breasts that are less than 25mms when compressed.
 Microcalcifications Identified on Mammography Microcalcifications Identified on Mammography |
| A cluster of suspicious pleomorphic calcifications are identified on a mammogram. These could represent either DCIS, or invasive carcinoma. A sterotactic biopsy is indicated, and a specimen radiograph of the biopsy follows.
28171 breast fx calcification mammogram mammography Courtesy Priscilla Slanetz MD DB |
 Specimen Biopsy Confirms That Correct Tissue has Been Biopsied Specimen Biopsy Confirms That Correct Tissue has Been Biopsied
Microcalcifications Present |
| 28173 breast fx calcification pathology Courtesy Priscilla Slanetz MD DB |
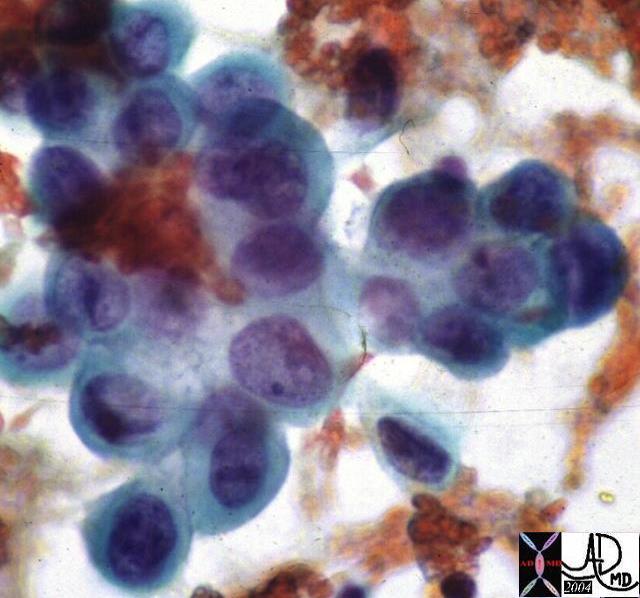 Cytopathology Specimen Infiltrating Ductal Carcinoma Cytopathology Specimen Infiltrating Ductal Carcinoma |
| This cytopathology specimen shows cells with high nuclear to cytoplasmic ratio characteristic of malignant tissue. Further characterization is difficult due to the limited amount of tissue that the pathologist has to work with.
13552 breast + dx infiltrating ductal carcinoma + cytopathology histopathology Courtesy Frank Reale MD DB |
Evaluation of Regional Nodes – The Axilla
The clinical examination of the axilla is not a sensitive nor specific procedure, and in the patient who is being prepared for surgery one of the key issues is to determine whether the regional nodes are positive for disease.
Preoperative evaluation with ultrasounded guided biopsy of suspicious nodes is a recent innovation that provides the surgeon with critical information. A negative biopsy is not usually helpful, though a positive biopsy may avoid extensive surgery.
Alternatively sentinel node imaging oof axillary nodes allows directed intraoperative sampling of lymph nodes by directing the surgeon to the node or nodal group that will most likely contain disease.
Prognostic Indicators
The prognosis and treatment of breast cancer are intimately related and multifactorial . At the simplest level, tumor size in the absence of nodal disease is an independent predictor. The prognosis detriorates with increasing size.
If the nodes are negative, then the averahge 10 year survival is 60-70%. If the nodes are positive the 10 year survival decreases to 20-30%.
Treatment Strategies
Treatment strategies are dependent on many factors primary of which, is the stage at diagnosis. The surgeon’s recommendation is based on the biopsy results and the extent of the disease.
The patients are divided into two broad groups at this stage; Those with disease that appears to be localized to the breast, and those that have definite disease that has spread beyond the breast. This broad division translates in to two therapeutic groups. The first group, where extent of disease is unknown, require surgery and evaluation of axillary nodes. The second, is the group that have locally inoperable disease or have spread beyond the breast and are inoperable. When the disease presents with obvious extension beyond the breast, where the skin regional lymph nodes or distant metastases are present, then surgery is not recommended.
We will follow the course taken by the first group of patients first andf then consider the therapeutic options available to the second group.
Surgical Options
In the absence of known regional involvement, surgery is the primary treatment since early stage patients can be cured by surgery alone.
The goal of surgery is;
complete resection of the tumor with negative margins and
regional pathologic staging of the tumor by establishing nodal involvement.
Resection of the Primary Tumor
The resection of the primary tumor may be by lumpectomy or mastectomy and is tailored according to the lesion, the breast size, the location of the tumor, the possibility of changing any of these factors preoperatively with chemotherapy, the patient’s needs and the skill of the surgeon and skill and experience of the overall team.
Breast conserving surgery in form of lumpectomy and radiotherapy lends itself best to small size tumors which are localized . It requires that the tumor is excisable with resulting negative margins and an acceptable cosmetic result. Thus a small lesion in a small breast may not be amenable for breast conserving therapy since the cosmetic result would not be acceptable. On the other hand a large lesion in close proximity to the skin or the chest wall may not be amenable for breast conserving therapy either. Sometimes such a lesion may be treated with chemotherapy before the surgery and if significant reduction in size is attained, then breast conserving surgery could become possible.
Lumpectomy infers resection of the tumor, ideally resulting in a 1cms disease free margin. Contraindication to lumpectomy includes multicentric diseasae, repeated positive margins, or a large primary tumor. Radiotherapy (XRT) is sometimes used as an adjunct to breast conserving surgery to prevent local recurrence, usually reserved for patients with invasive ductal carcinoma or DCIS, tumor size <3cms, negative surgical margins and negative nodes.
A total mastectomy is the complete removal of breast tissue, without necessarily removing any lymph nodes.
A radical mastectomy is the removal of the breast tissue with en bloc removal of the pectoralis muscle and and axillary nodes.
A modified radical mastectomy is a total mastectomy with axillary node dissection.
Each of these surgeries is in some way dependant on the local disease, and the suspicion or knowledge of nodal disease. Either way nodal involvement has to be established pathologically, and there are multiple mechanisms of trying to establish involvement.
Other Surgery
Breast reconstruction surgery:
Reconstruction of breast after mastectomy is done either at time of surgery or months after primary surgery. Some women choose not to undergo reconstruction.
Prophylactic mastectomy:
Some women choose to undergo prophylactic mastectomy of opposite breast at the primary surgery of affected breast. Some young patients with high risk mutations and strong family history may choose to undergo prophylactic mastectomy.
Bilateral ovary removal:
Some women may undergo ovary removal to reduce the amount of circulating estrogen thus reducing risk.
Nodal Evaluation
Anatomy of the Lymph Nodes
The breast has a very rich supply of lymphatics, with the dominant flow being toward the axilla. The lymphatics originate in the lobules, and they accompany the veins and ductal systems coursing toward the nipple and areola. An extensive network develops in the subareolar region called the subareolar plexus.
There are about 35 nodes that drain the breast and they are basically divided into 4 regions. The axillary nodes are the largest group and they drain about 75% of the breast. The other major groups include the parasternal group, the infraclavicular group, and the supraclavicular group.
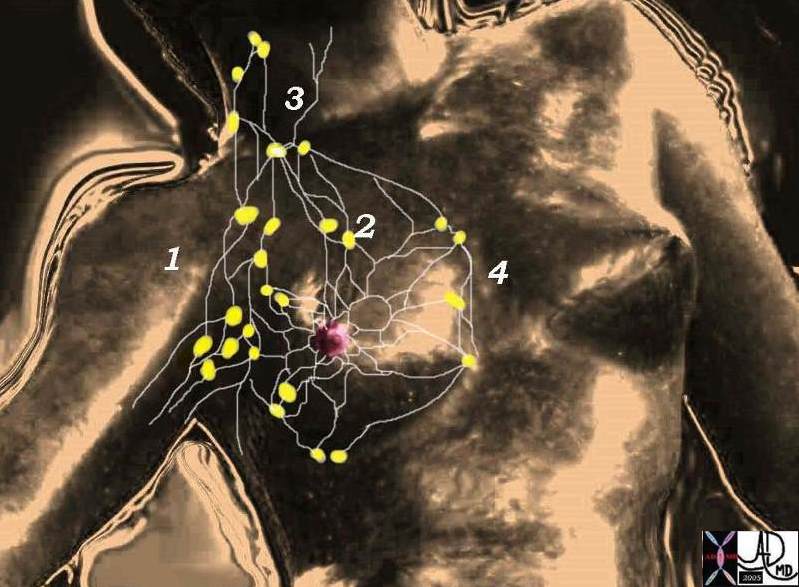 Lymphatic supply of the breast Lymphatic supply of the breast |
| There are 4 basic groups of lymph nodes the largest of which are the axillary group (1) which account for 75% of the lymphatic drainage. The other groups are the infraclavicular (2), supraclavicular (3), and parasternal (4) groups of nodes. Davidoff MD. 78378pb06l05 |
Subgroups of the axillary system of nodes
There are subgroups of the axillary nodes that mostly lie alongside the veins described above. The first of these is called the anterior pectoral group (aka low axillary nodes), consists of about four nodes, and are distributed alongside the lateral thoracic vessels on the lateral border of the pectoralis muscles. The nodes that lie along the axillary vein are called the central axillary nodes. The nodes that lie alongside the subclavian vein are called the subclavian nodes.
There is a set that is not necessarily associated with any specific vein that lies deep to both pectoral muscles under the breast. This group is called the deep fascial system and they drain into set of nodes between the pectoral muscles called Rotter’s nodes. Subsequently some of these nodes will drain into the subclavian nodes and some will drain medially into the internal mammary system which finally drains into the mediastinal nodes.
The other regions described including the infraclavicular, supraclavicular and internal mammary lymphatic drainage are less dominant sites.
Even more uncommon is drainage to the infradiaphragmatic nodes.
It should also be noted that there are intramammary lymph nodes that sometimes masquerade as a breast mass since they have features. On mammography they are typically reniform in shape, have a fatty or radiolucent notch, are less than 1 cm in size and are usually in upper outer quadrant. Cyrlak describes a case of an enlarged intramammary node that turned out to be a site of metastatic involvement of breast carcinoma.
Applied Anatomy
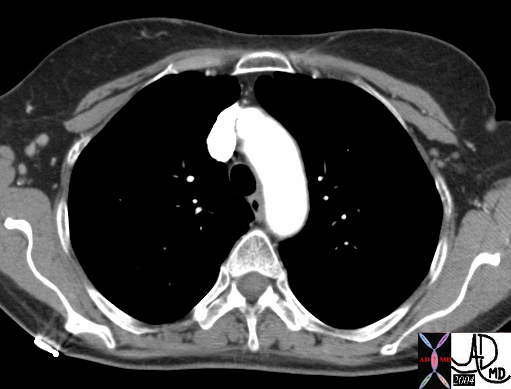
 Lymph nodes Lymph nodes
|
| This is a CT scan of a 62year normal female that shows axillary nodes on the right. These nodes are considered “level 1” lymph nodes in surgical terminology (yellow). They lie lateral to the pectoral muscles. On the left the nodes in orange are level 2 nodes and they lie deep to both pectoral muscles. They belong to the group called the deep fascial system. Courtesy Ashley Davidoff MD 43843 43843b02 |
The staging and hence the management of breast carcinoma depends to a large extent on whether the lymph nodes of the breast are involved. Most of the search for disease is directed to the axillary group of nodes. Clinical examination is the first step, mammographic findings the second, sentinel node evaluation using radiopharmaceuticals the third, and surgery and microscopic evaluation the fourth. In cancers involving the medial breast tissue, when the axillary nodes are negative the internal mammary chain is involved in only about 15% of cases, and in lateral cancers the internal mammary nodes are only involved in about 5% of cases.
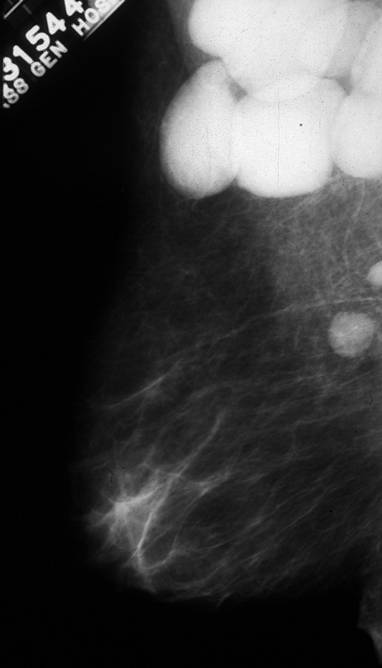
Lymph nodes |
| This mammogram reveals large and dense nodes in left axilla. This patient had leukemic involvement of these nodes. Courtesy Priscilla Slanetz MD MPH 43000 |
The sentinel node is defined as the first lymph node to drain a region of the breast and in the setting of breast carcinoma would be the first node to contain metastatic disease.
The Sentinel Node Evaluation
If the nodes are clinically negative then the approach is to evaluate the sentinel node which is the node or group of nodes that drain from the breast to the axilla. Sentinel node identification is evaluated by injecting a small dose of low level radioactive technetium 99 and isosulfan blue dye into the skin in the region of the tumor prior to surgery. A period of between 1-8 hours allows the blue dye and tracer to be taken by the lymphatic system to the regional nodes. A hand held gamma ray counter allows the surgeon to identify the sentinel node. A small incision is made over the radioactivity and between 1 and 3 nodes are removed and given to the pathologist. By this means only limited dissection of the lymph nodes is attained with reasonable certainty that if the sentinel nodes are negative then nodes more distal are negative. If it the sentinel node is positive the tumor is upstaged and further dissection of the lymph nodes is required. In the past all axillary nodes would have been removed with consequent morbidity, including significant swelling of the arm from lymphedema and consequent cellulitis.
Axillary Node Evaluation
Surgical approach to lymph node dissection and resection requires dividing the nodes into 3 levels, going in general from lateral to the breast, to deep and under the breast and pectoral muscles, to deep and medial to the breast. More specifically, a level I dissection is bordered by the axillary vein superiorly, the lateral border of the pectoralis major and minor muscles medially, and the latissimus dorsi muscle laterally. A level II dissection includes nodes below the pectoralis minor muscle, and a level III includes nodes medial to the pectoralis minor muscle and below the costoclavicular ligament. In general if a patient requires lymph node dissection, resecting nodes from Level I and II is considered adequate. Level III nodes are only removed if they are suspicious .
It is now reasonable to discuss staging disease in both groups of patients. Those who were subjected to surgery who now are post op with knowledge of nodal status, and those that were considered inoperable due to extensive inoperable local disease or due to known regional or distant disease.
Staging
Cancer stage is based on the size of the tumor, whether the cancer is invasive or non-invasive, whether lymph nodes are involved, and whether the cancer has spread beyond the breast.
Stage 0
Stage 0 is used to describe non-invasive breast cancers, such as DCIS and LCIS. In stage 0 the cancer is contained and there is no evidence of cancer spreading to normal surrounding tissue.
Stage I
Stage I describes invasive breast cancer wherein there is evidence of cancer cells are invading the surrounding normal tissue
- the tumor measures up to 2 centimeters, and
- no lymph nodes are involved
Stage II
Stage II is divided into subcategories known as IIA and IIB.
Stage IIA describes invasive breast cancer in which:
- no tumor can be found in the breast, but cancer cells are found in the axillary lymph nodes (the lymph nodes under the arm), OR
- the tumor measures 2 centimeters or less and has spread to the axillary lymph nodes, OR
- the tumor is larger than 2 centimeters but not larger than 5 centimeters and has not spread to the axillary lymph nodes
Stage IIB describes invasive breast cancer in which:
- the tumor is larger than 2 but no larger than 5 centimeters and has spread to the axillary lymph nodes, OR
- the tumor is larger than 5 centimeters but has not spread to the axillary lymph nodes
Stage III
Stage III is divided into subcategories known as IIIA, IIIB, and IIIC.
Stage IIIA describes invasive breast cancer in which either:
- No tumor is found in the breast. Cancer is found in axillary lymph nodes that are clumped together or sticking to other structures, or cancer may have spread to lymph nodes near the breastbone, OR
- the tumor is 5 centimeters or smaller and has spread to axillary lymph nodes that are clumped together or sticking to other structures, OR
- the tumor is larger than 5 centimeters and has spread to axillary lymph nodes that are clumped together or sticking to other structures
Stage IIIB describes invasive breast cancer in which:
- the tumor may be any size and has spread to the chest wall and/or skin of the breast AND
- may have spread to axillary lymph nodes that are clumped together or sticking to other structures, or cancer may have spread to lymph nodes near the breastbone
- Inflammatory breast cancer is considered at least stage IIIB.
Stage IIIC describes invasive breast cancer in which:
- there may be no sign of cancer in the breast or, if there is a tumor, it may be any size and may have spread to the chest wall and/or the skin of the breast, AND
- the cancer has spread to lymph nodes above or below the collarbone, AND
- the cancer may have spread to axillary lymph nodes or to lymph nodes near the breastbone
Stage IV
Stage IV describes invasive breast cancer in which:
- the cancer has spread to other organs of the body — usually the lungs, liver, bone, or brain
“Metastatic at presentation” means that the breast cancer has spread beyond the breast and nearby lymph nodes, even though this is the first diagnosis of breast cancer. The reason for this is that the primary breast cancer was not found when it was only inside the breast. Metastatic cancer is considered stage IV.8
Additional staging information
Terms such as “early” or “earlier” stage, “later,” or “advanced” stage breast cancer are not medically precise but are often used by medical professionals loosely.
following is the break up of early versus advanced.8
Early stage
- Stage 0
- Stage I
- Stage II
- Some stage III
Later or advanced stage
TNM
TNM (status of Tumor, Nodess and Metastases), is another method of defining and classifying breast carcinoma.
Definitions for classifying the primary tumor (T) are the same for clinical and for pathologic classification.
Primary tumor (T) . 9
- TX: Primary tumor cannot be assessed
- T0: No evidence of primary tumor
- Tis: Intraductal carcinoma, lobular carcinoma in situ, or Paget disease of the nipple with no associated invasion of normal breast tissue
- Tis (DCIS): Ductal carcinoma in situ
- Tis (LCIS): Lobular carcinoma in situ
- Tis (Paget): Paget disease of the nipple with no tumor.
- T1: Tumor not larger than 2.0 cm in greatest dimension
- T1mic: Microinvasion not larger than 0.1 cm in greatest dimension
- T1a: Tumor larger than 0.1 cm but not larger than 0.5 cm in greatest dimension
- T1b: Tumor larger than 0.5 cm but not larger than 1.0 cm in greatest dimension
- T1c: Tumor larger than 1.0 cm but not larger than 2.0 cm in greatest dimension
- T2: Tumor larger than 2.0 cm but not larger than 5.0 cm in greatest dimension
- T3: Tumor larger than 5.0 cm in greatest dimension
- T4: Tumor of any size with direct extension to (a) chest wall or (b) skin, only as described below
- T4a: Extension to chest wall, not including pectoralis muscle
- T4b: Edema (including peau d’orange) or ulceration of the skin of the breast, or satellite skin nodules confined to the same breast
- T4c: Both T4a and T4b
- T4d: Inflammatory carcinoma
Regional lymph nodes (N)
- NX: Regional lymph nodes cannot be assessed (e.g., previously removed)
- N0: No regional lymph node metastasis
- N1: Metastasis to movable ipsilateral axillary lymph node(s)
- N2: Metastasis to ipsilateral axillary lymph node(s) fixed or matted, or in clinically apparent ipsilateral internal mammary nodes in the absence of clinically evident lymph node metastasis
- N2a: Metastasis in ipsilateral axillary lymph nodes fixed to one another (matted) or to other structures
- N2b: Metastasis only in clinically apparent ipsilateral internal mammary nodes and in the absence of clinically evident axillary lymph node metastasis
- N3: Metastasis in ipsilateral infraclavicular lymph node(s) with or without axillary lymph node involvement, or in clinically apparent ipsilateral internal mammary lymph node(s) and in the presence of clinically evident axillary lymph node metastasis; or, metastasis in ipsilateral supraclavicular lymph node(s) with or without axillary or internal mammary lymph node involvement
- N3a: Metastasis in ipsilateral infraclavicular lymph node(s)
- N3b: Metastasis in ipsilateral internal mammary lymph node(s) and axillary lymph node(s)
- N3c: Metastasis in ipsilateral supraclavicular lymph node(s)
* [Note: Clinically apparent is defined as detected by imaging studies (excluding lymphoscintigraphy) or by clinical examination or grossly visible pathologically.]
Pathologic classification (pN)*
-
- dissection but not clinically apparent** (If associated with more than three positive axillary lymph nodes, the internal mammary nodes are classified as pN3b to reflect increased tumor burden.)
Distant metastasis (M)
- MX: Presence of distant metastasis cannot be assessed
- M0: No distant metastasis
- M1: Distant metastasis
AJCC Stage Groupings
Stage 0
Stage I
Stage IIA
- T0, N1, M0
- T1*, N1, M0
- T2, N0, M0
Stage IIB
Stage IIIA
- T0, N2, M0
- T1*, N2, M0
- T2, N2, M0
- T3, N1, M0
- T3, N2, M0
Stage IIIB
- T4, N0, M0
- T4, N1, M0
- T4, N2, M0
Stage IIIC**
Stage IV
Management After Staging
Following are the modalities that are employed in management of breast cancer
The treatment may be in either of the following sequences in most cases.
- surgery → radiation → possible hormonal therapy
- surgery → chemotherapy → radiation → possible hormonal therapy
- Chemotherapy, targeted therapy, or hormonal therapy → surgery → radiation → possible hormonal therapy.
- Chemotherapy – possibly radiation for palliation
Chemotherapy:
Chemotherapy is recommended on the basis of findings after surgery, relating to tumor size and lymph node involvement. In addition to stage of disease hormonal receptor status as well as Her 2 neu status. Chemotherapy affects rapidly dividing cancer cells and therefore may also affect cells of blood, hair, and gastrointestinal tract. The most common side effects of chemotherapy are hair loss, low blood counts, nausea and vomiting. Monoclonal antibody against Her 2 neu is used if the receptor is positive in tissue. Anti hormone medications are used in case hormone receptors are positive.
Chemotherapy has played a major role in cancer treatment for half a century. The cancer drugs work by killing the rapidly dividing cells. Since the cancer cells are rapidly dividing they are affect other rapidly dividing cells and tissues. However there are certain normal cells in the body that have very rapid turnover and are affected by chemotherapy such as bone marrow and gastrointestinal epithelium and hair follicles. Therefore patients receiving chemotherapy may suffer decrease blood cell counts, hair loss and diarrhea. Unlike radiation and surgery which are much more localized, chemotherapy can target cancer cells that have escaped from the original tumor site and may cause metastasis.
Chemotherapy may be used for following goals depending upon staging and type of cancer.
- Cure of the cancer by eliminating all cancer cells in the body, even when cancer is widespread
- Prolong life by controlling cancer growth and spread
- Relieve symptoms and enhance quality of life
- In conjunction with radiation they can act as radiosensitizers, making cancer cells more susceptible to radiation
- Following surgery to enhance results. This is called as adjuvant therapy.
- Prior to surgery or radiation making disease more amenable to treatment and is called as neoadjuvant therapy.
Radiation:
Radiation therapy is important in all stages of breast cancer. It is important from stage 0-III for treatment of breast cancer. Radiation attempts to destroy the cancer cells left behind after lumpectomy or mastectomy. It may have a role in palliation in stage IV. Side effects of radiation are usually limited to the localized to the area of radiation.
Hormonal therapy:
Hormonal therapy is used in patient with hormone receptor positive cancers. Hormonal therapy medicines treat hormone-receptor-positive breast cancers in two ways:
- by lowering the amount of the hormone estrogen in the body
- by blocking the action of estrogen in the body
The estrogen is produced primarily by ovaries in a woman’s body and therefore women who are premenopausal may undergo bilateral ovary removal. There are two drug classes which are used for hormonal therapy.
Selective estrogen receptor blocker, blocks the selected estrogen receptors in breast tissue but may bind and activate estrogen receptors in other part of the body.
Aromatase inhibitors, block production of estrogen. Since they cannot inhibit estrogen production in ovaries, they are therefore are only effective in postmenopausal women.
Targeted Therapies:
There are three types of targeted therapies
Trastuzumab: It is a monoclonal antibody that blocks her 2 Neu a receptor found in some breast cancer. Blocking the receptors helps block the proliferation of cancer cells.
Lapatinib: It is also a her 2 neu receptor blocker.
Avastin: It is a vascular endothelial growth factor block which prevents the proliferation of blood vessels on which cancer cells depend to multiply.
Principles of Treatment of Breast Cancer
Principles of treatment relate to the staging of tumor:
Stage 0:
Refers to non invasive cancer or DCIS. DCIS is optimally treated with mastectomy. Breast conserving surgery i.e. lumpectomy and radiation is also an option. Lymph node dissection is usually not done. However sentinel lymph node biopsy can be carried out. Tamoxifen may be used for chemoprevention in hormone receptor positive tumor
Stage I:
Most stage one breast cancers are treated with mastectomy. Although a majority of breast cancer may be treated by lumpectomy and followed by radiation. Tumors larger than 1 cm have higher recurrence rate. Hormone treatment is recommended in cancers which are hormone receptor positive to reduce recurrence. Tamoxifen reduces recurrence risk by 40-50%. Recurrence score may be calculated using gene based prognostic score and a recommendation if chemotherapy is needed or not is made.
Stage II:
Stage II breast cancers are treated by mastectomy or breast conserving therapy as appropriate with sentinel axillary lymph node dissection or extensive axillary lymph node dissection as appropriate. Chemotherapy confers a higher absolute risk reduction and therefore recommended. Radiation is recommended following mastectomy to patients who have 4 or more positive lymph nodes. Hormone therapy is recommended for hormone receptor positive tumor.
Stage III:
Patients with stage III breast cancer can be classified into two general categories. Some have large tumors, without skin involvement or fixation to deeper tissues, which are clearly operable. Others have neoplasms that are considered inoperable because skin involvement or fixation to the underlying chest wall precludes total resection with clean margins. Some stage III tumors can be treated as stage II. Inoperable stage III tumors may require chemotherapy/radiation followed by radiation. Hormonal therapy is recommended upfront if chemotherapy cannot be administered. Radiation therapy is considered a cornerstone of local therapy.
Stage IV:
Metastatic disease should be considered and approached as a chronic illness. The goals of therapy are to palliate symptoms, prolong life, and, if possible, achieve a long term, disease-free state. The options are from cytotoxic chemotherapy, hormonal therapy and targeted therapy depending on Her 2 positive status. Patient who are in poor general health or have other serious medical condition may be treated with hormonal therapy if cancer is hormone receptor is positive or supportive measure.
The treatment of breast cancer is complex and needs to be tailored to each patient, and a multidisciplinary approach by an oncology team is extremely useful.
Oncology Team
The team of subspecialists consist of the following members.
The radiologist is responsible for interpreting the mammogram, ultrasound or MRI, and then in the patient with the non palpable mass is instrumental in the biopsy of the abnormality. In the staging evaluation of metatstattic disease, evaluation by CTscan, MRI, PET scan, or bone scan is in the pervue of the radiologist.
The pathologist particpates both preoperatively as well as postoperatively. In the preoperative patient grading of the tumor, identifying the type of breast cancer, hormone receptor status, as well as Her 2 neu status. During and after surgery evaluation of the margins of the primary tumor and involvement of the nodes enables the post operative staging and prognostication of the tumor.
Surgeons help in deciding which type of breast surgery would be most apprpriate for a given situation, and then executing the surgery in the operating room and deciding which nodes to evaluate or remove.
The radiation oncologist: particpates in the control of local and regional disease as well as palliation of symptoms in advanced and metastatic disease.
The oncologists coordiate the care, integrate the care and direct chemotherapy including the type of chemotherapy, and the utility of hormonal therapy or targeted therapy. Oncologists usually monitor patient on long term basis to assess recurrence and prompt management of recurrent disease.
Nurses provide care to the patient thorough all the steps of treatment in the operating room, in administering chemotherapy in oncology unit, and as confidants and support for those who need it. Nurses undergo special training to assist in the OR and to administer chemotherapy.
Multiple other technologists are involved with the patient care, including radiology technologists, radiation therapy technologists, and lab technologists. Patients often develop important and intimate relationships with the technologists and nurses.
Social workers deal with issues such as financial hardships, transport and providing assistance in placement to rehabilitation centers or hospice.
Nutritionists help in supporting nutritional status of the patients especially with side effects associated with treatment such as mucositis.
Hospice nurses: Help in care of end of life issues in patients who are dying due to their disease
Psychological Aspects
Diagnosis of breast cancer is devastating news for most patients, and runs deep and profound. Along with dealing with a potentially life threatening disease most women worry about the effect of treatment on their appearance. It is important to address these issues at the outset as well as periodically during duration of treatment. If required an appointment should be made to seek assistance from a psychologist or psychiatrist. Support groups are extremely helpful allowing the patient to express deep feelings that are common to many woman with the same disease allowing for some cathartic and therapeutic beneficial effects.
Critical illness leaves no aspect of life untouched. Diagnosis of cancer, as well as stress associated with cancer, is trying for most patients. The initial period is dominated by medical aspects of obtaining diagnostic information and undertaking primary or adjuvant therapy. Fear anxiety and pain are common at this stage. Coping with therapy requires all the adaptive energies of most patients. Following treatment, there is an extended period when patients adjust to the follow up after acute treatment. Providing psychosocial support to patients during these phases is essential.
Families and friends are an important source of psychological support. Social workers are also an important source of support to patients. For patient in terminal stages of cancer patient may have fear, pain and felling of helplessness and loss of control over their lives. It is important allow patients to take control of their lives and treatment decisions. Patients also worry about being a burden on their families. Therefore it is important to allow the patients to make decisions about where they want to be treated.
Family
Breast cancer not only affects the patients but also their families. A number of patients may have young families and may find it difficult to talk to their young children about their diagnosis. Many sources such as American cancer society may be resources that patient can refer to. It is important to prepare the family members such as spouses and young children as to how the treatment will affect daily routine.
Family members undergo considerable psychological turmoil when a loved one is diagnosed with cancer. The distress may at times be considerable enough to affect the patient. The best way to deal with these issues is to educate and provide support to family members as sometimes they may be the primary caregiver of the patients. Some patients may not like the family members to be involved in their care.
Social
Diagnosis of cancer affects all aspects of a patient’s life. A number of patients continue to work full time during treatment while others choose to take time off. Most patient struggle with the impact of their diagnosis and worry about their appearance and economic impact of the treatment. Patients can and should continue their normal life as much as possible.
Their every day life is affected and they may have questions such as …. Will I lose my hair? Will my appearance change? How will my social life be impacted? Will I be able to go out and enjoy a meal in the restaurant?
Most patients are able to work through their diagnosis and treatment. The ability to adapt to the disease, depends on the patient’s age and performance status prior to beginning the therapy. The patient may feel conscious about hair loss while going out in public, therefore an effort should be made to address this issue at the outset. The oncology nurse will usually go over with the patient about wigs and uses. Most patients are able to go out and spend time with the family and friends however if they are neutropenic but it is recommended that they avoid crowded places and certain foods. Patients undergoing transplant may be asked to make lifelong dietary changes for example; to eat only well done meats.
It is important to address issues such as fertility, future pregnancy and contraception
Nutritional
Nutrition is important as good nutrition help keeps the body strong so that treatment can be administered in a timely fashion. A nutritionist may help in formulating a healthy diet — one with a variety of foods that includes lots of fruits and vegetables and regular protein — which gives the reserves of nutrients that patients need to keep their strength up while being treated for breast cancer. These reserves also help rebuild body’s tissues and keep immune system strong to help fight off infection. Plus, a healthy diet can help in managing treatment side effects. There is evidence that some cancer treatments actually work better in people who are eating enough calories and protein. During the breast cancer treatment, it’s more important than ever that patients eat a healthy diet.
Patients may lose weight and appetite secondary to the cancer. Challenges to nutrition are as a result of chemotherapy induced nausea, vomiting , mucositis and diarrhea and radiation induced mucositis and diarrhea . Patients may become severely dehydrated and their nutrition may suffer severely. It is important to take a good nutritional history as well as history of any adverse effect that may severely impact oral intake. A focused exam should include looking for mucositis. Patients may do well with nutritional shakes and soft diet while these symptoms abate. A nutritional consult is often helpful in determining the available options.
New Advances
The field of cancer is changing everyday. There are new and improved drugs that are coming out, or in pipeline;
Targeted therapy: These drugs target a particular abnormality or receptor in the cancer cell, and therefore normal cells may be spared from chemotherapy.
Radioimmunotherapy: radioactive ligands such as iodine are tagged on to antibody targeting a specific molecule and thus allow for targeted radiation and immunotherapy
Defining risk and response in the patients: Scientists are looking for parameters that may predict if a tumor will respond to a particular chemotherapy and therefore allowing the patient to be treated only if they have responsive disease.
End of Life Issues
End of life issues are major part of caring for a cancer patient. It is important to help a patient and family understand their prognosis and help them cope with their terminal illness.
Hospice: Hospice care is a philosophy about care of people who are nearing the end of their lives. Hospice care is designed to relieve pain, or other symptoms, and provide as much quality time as possible with family and friends. The focus of hospice care is no longer on curing or treating the underlying disease. The goal of hospice care is to provide the highest quality of life for whatever time remains.
It is important to emphasize to the patient that by discussing hospice you are not abandoning them. Instead you are helping them focus on care and spending time with the family.
There are tow types of hospice care: home hospice and residential hospice in an institution. Any one of these may be chosen depending upon patient’s family situation and social support.
A hospice care team consists of following:
Doctor: provides a treatment plan tailored to the patient and as well as performs a supervisory role.
Hospice nurses are trained professional who are expert in providing pain and symptom relief to a terminally ill patient.
Home health aides: provide care to a patient.
Prognosis
The prognosis of breast cancer varies depending upon type of cancer, age and performance status of the patient, high risk features in histopathological profile.
The following is stagewise overall prognosis
Stage 0 -DCIS
Is usually cured by definitive surgery and recurrence risk is low
Early stage breast cancer:
For cancer less than 2 cm and lymph node negative as well as intermediate grade pathology, 10 year survival is 85-89%. Cancer that are less than 2 cms with high grade features 10 year survival is 70-78 %
Advanced or late stage breast cancer;
Cancers that are large in size with lymph node involvement prognosis varies a lot from 35-78% depending on the size, grade of the lesion and lymph node involvement
Metastatic breast cancer: prognosis is poor in metastatic stage, it is not curable and only 23% of women are alive at 5 years.
Conclusion
Breast cancer represents a significant challenge in terms of diagnosis, prognosis as well as treatment. There are multiple variables that determine the aggressiveness of the disease. In a particular the treatment of one patient may be different than another woman with similar stage and there are multiple algorithms defining optimal treatment in a certain patient. Though breast cancer is serious disease, the treatment options have increased over last few years. Chemotherapy side effects are less and radiation is much more precise. Breast cancer is a disease that can be cured in early stages and can be controlled in advanced stages. Women may continue to lead their lives even with breast cancer.
Red Flags
The following conditions should warrant concern and a comprehensive emergency room evaluation in the oncology patient, as they may be signs of a life-threatening condition:
Fever
New or sudden chest, back or abdominal pain
Night sweats and extreme fatigue
Pre-syncope or syncope
Severe eye or headache
Change in mental status, including but not limited to confusion and lethargy.
Focal weakness.
Productive cough
Hemoptysis
Tachypnea .
Tachycardia,
Bony pain
Fall with or without head trauma
References.
References.
- Jemal A, Siegel R, Ward E, et al.Cancer Statistics, 2006.CA Cancre: J Clin. 2006;56(2):106–130
- Familial breast cancer: collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease.Lancet. 2001;358(9291):1389–1399.
- National Cancer Institute. Breast Cancer Risk Assessment Tool
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation.JAMA. 1999;281(23):2189–2197.
- Cauley JA, Norton L, Lippman ME, et alWhole-Body PET Scans Have High False Positive Rates for Breast Cancer” by C.E. Carr and others, American Society of Clinical Oncology Annual Meeting, June 2006,
6.Breast Imaging Reporting and Data System (BI-RADS). 4th ed. Reston, Va.: American College of Radiology, 2003.
- Radiology Infounder the Auspices of the American College of Radiology
- Breastcancer.org
- AJCC Cancer Staging Manual byFrederick L. Greene, David L. Page (Editor), et al
(6th Edition) (Springer)
- Stockler M, Wilcken NR, Ghersi D, et al. Systematic reviews of chemotherapy and endocrine therapy in metastatic breast cancer.Cancer Treat Rev.2000;26(3):151–168.
- Singhal H, Gohel M.S., Kaur, K, Thomson, S Breast Cancer Evaluation eMedicine
12 Swart R MD PhD Downey L MD, Lang J MD, Thompson P PhD, Breast Cancer :Treatment eMedicine
13 Kopans D B MD Breast Imaging 3 rd Edition Lippincott Williams and Wilkins
14 Echevarria JJ, Lopez-Ruiz JA, Martin D, Imaz I, Martin M. Usefulness of MRI in detecting occult breast cancer associated with Paget’s disease of the nipple-areolar complex. Br J Radiol. 2005;77(924):1036-9 PMID:15569646
15 Fajardo LL, Pisano ED, Caudry DJ, Gatsonis CA, Berg WA, Connolly J, Schnitt S, Page DL, McNeil BJ,
Stereotactic and sonographic large-core biopsy of nonpalpable breast lesions: results of the Radiologic Diagnostic Oncology Group V study.
. Acad Radiol. 2006;11(3):293-308 PMID:15035520
15 Haagensen CD, Lane N, Lattes R, Bodian C. Lobular neoplasia (so-called lobular carcinoma in situ) of the breast. Cancer 1978 Aug;42(2):737-69
16 Harminder K. Gill, MD and Wendie A. Berg, MD, PhD. Invasive Lobular Carcinoma Radiology. 2001;221:132-136.
17 Hilleren, IT Andersson, K Lindholm and FS Linnell Invasive lobular carcinoma: mammographic findings in a 10-year experience Radiology, Vol 178, 149-154,
18 Garnett, S Wallis M, and Morgan G
Do screen-detected lobular and ductal carcinoma present with different mammographic features? Br. J. Radiol., January 1, 2009; 82(973): 20 – 27.
Questions (20 MCQ)
1) Which of the following is correct?
Breast cancer
- a) Is the most common cancer in the world
- b) Is the most common cancer in men
- c)Is not as common as lung cancer
- d) Is only a cancer of women
- e) Is not really a true cancer
2) Which of the following is correct?
- a)Family history is only important after the age of 40
- b)Family history on both the mothers side and fathers side are importnt
- c)Genetics plays a dominant role in breast cancer
- d)Breast cancer is highest in African American women
- e)Breast cancer is as frequent in first degree relatives as the general population
3) Regarding the hormonal aspects of breast cancer
- a)Progesterone exposure is most important factor
- b)Estrogen exposure plays only a small part in hormonal theories
- c)Hormonal replacement therapy has no relevance to the development of breast cancer and is recommended for all post menopausal women.
- d)Gender is the most important risk factor in the development of breast cancer and occurs 100 more frequent in men than women.
- e)The shorter the exposure to estrogen the higher the likelihood of breast cancer
4) Regarding diet and breast cancer?
- a)Vegetables have no estrogens and have no beneficial effect
- b)Vegetables have phytoestrogens which compete with the bodies estrogens and reduce risk of cancer
- c)Alcohol helps prevent breast cancer
- d)Obesity helps prevent breast cancer
- e)Anorexia helps prevent breast cancer
5) The terminal duct lobular unit(TDLU)
- a)Is the same as the breast lobule?
- b) Is the support tissue of the breast?
- c)Is the most metabolically active unit of the breast?
- d)Is present in the breast only when the patients cancer is terminal
- e)Is the place where cancerous sarcomas arise
6) Which of the following is correct?
- a) There are 10-20 lobules in the mature breast
- b)There are 10-20 acini in the pregnant breast
- c)There are 10-20 TDLU’s in the lactating breast
- d)There are 10-20 ductules in the breast
- e)The there are 10-20 lobes in the breast
7) Regarding the anatomy of the breast?
- a)There are 10-20 ducts in each breast opening separately on the nipple
- b)The axillary tail of Spence represents the suspensory ligaments in the breast
- c)Cooper’s ligaments are seen only in the breasts of women from Cooper’s town
- d)Cooper’s ligaments are really extra areas of glandular tissue in the axilla
- e)All breasts consists dominantly of glandular tissue
8) Regarding in situ disease of the breast?
- a)DCIS and LCIS have the same behavioral patterns
- b)DCIS is associated with calcifications on mammography
- c)LCIS is associated with calcifications on mammography
- d)Calcifications are best seen on ultrasound
- e)The basement membrane is invaded in the in situ carcinomas
9) Which of the following is correct?
- a)DCIS occurs in 70-95% of women at autopsy
- b)LCIS arises from the supportive tissue
- c)DCIS arises from the supportive tissue
- d)DCIS is multicentric and more often involves the other breast
- e)These lesions are both always active and never involute, always becoming invasive cancer
10) Invasive Ductal Carcinoma
- a) Is not associated with calcifications on mammography
- b)Is only associated with calcifications in the phyllodes subtype
- c)Is only malignant when calcifications are present
- d)Is the most common form of breast carcinoma
- e)Is usually a very soft tumor
11) Invasive Ductal Carcinoma
- a)Is really a type of sarcoma and is also called a Phyllodes tumor
- b)Is an in situ carcinoma
- c)Can undergo necrosis and a cheesy material can be extruded from the ducts
- d)Does not undergo necrosis and peau d’orange is extruded from the ducts
- e)Nipple retraction never occurs
12) Invasive Ductal Carcinoma
- a)Is the least common breast carcinoma and carries the worst prognosis
- b)Is the least common breast carcinoma and carries the best prognosis
- c)Is the most common breast carcinoma and carries the best prognosis
- d)Is the most common breast carcinoma and carries the worst prognosis
13) Invasive Lobular Carcinoma
- a)Is always palpable clinically
- b)Has a desmoplastic, fibrotic nature and always feels hard to palpation
- c)Is characterized by tubular and pleomorphic calcifications
- d)Is characterized by its bilaterality
- e)Is the most common form of breast cancer
14) Regarding invasive lobular carcinoma?
- a)Ultrasound is the most sensitive imaging modality
- b)CT scan is the most sensitive imaging modality
- c)Mammography is the most sensitive imaging modality
- d)MRI is the most sensitive imaging modality
- e)PET scan is the most sensitive imaging modality
15) Inflammatory breast carcinoma?
- a)Is diagnosed by finding malignant cells in the skin biopsy
- b)Is diagnosed by finding inflammatory cells in the skin biopsy
- c)Is not really a carcinoma but an inflammatory condition of the breast
- d)Is treated with surgery and then steroids to reduce the inflammation
- e)Is treated with surgery and then antibiotics to reduce the inflammation
16) Regarding mammography
- a)Is being superseded by MRI as the study of choice for breast cancer
- b)Annual mammography is recommended for all women starting age 40
- c)Is safe in the first trimester of pregnancy
- d) Has no effect on cancer mortality
- e)False negative rate is unacceptably high
17) Regarding ultrasound
- a) Annual ultrasound is recommended for all women starting age 40
- b)Has no place in breast cancer evaluation
- c)Is used to guide core biopsy of a lesion
- d)Is unable to distinguish between a cyst and a solid lesion
- e)Is contraindicated in pregnancy
18) Regarding surgery
- a)All patients with breast cancer should have the lesion surgically removed
- b)Patients with stage IV disease require surgery
- c)Cancer of the breast is an aggressive disease and therefore requires radical mastectomy
- d)Cancer of the breast is not such an aggressive disease and therefore only requires lumpectomy
- e)The goal of surgery is complete resection of the tumor, and establishing the involvement of regional nodes
19) Regarding chemotherapy?
- a)Always precedes surgery
- b)Sometimes used to follow surgery to enhance results
- c)Is only effective in treating macroscopic disease
- d)Is only effective in treating local disease at both the macroscopic and microscopic level
- e)Cannot relieve symptoms or enhance the quality of life and in fact does the oppposite
20) Regarding radiotherapy
- a)Is used for local control of disease that may have been left behind after surgery
- b)Is not used in stage IV disease
- c)Is used prophylactically in the spine and liver which are common sites for metastases
- d)There are usually no local side effects
- e)Side effects are commonly systemic and include nausea, vomiting, and loss of hair




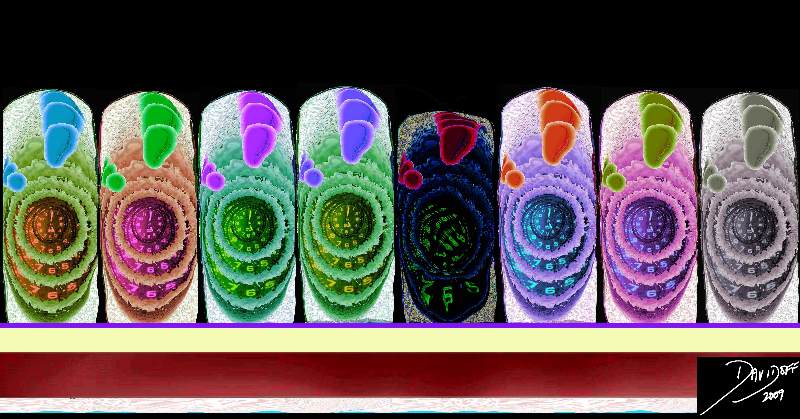

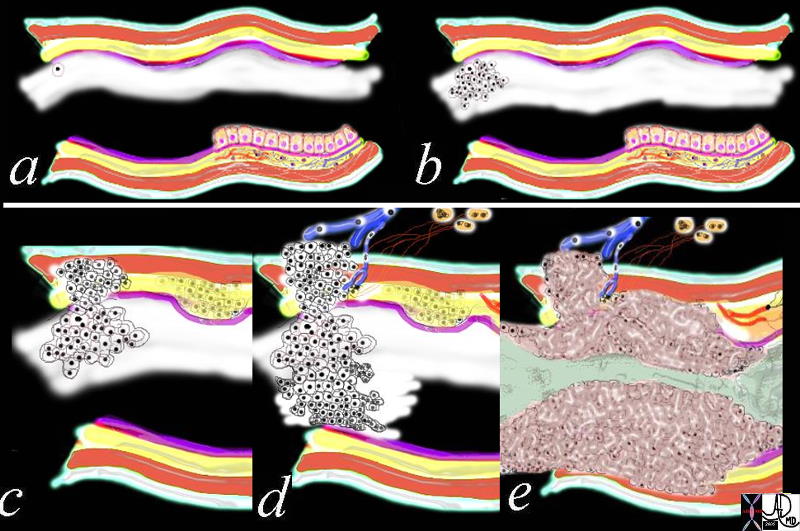
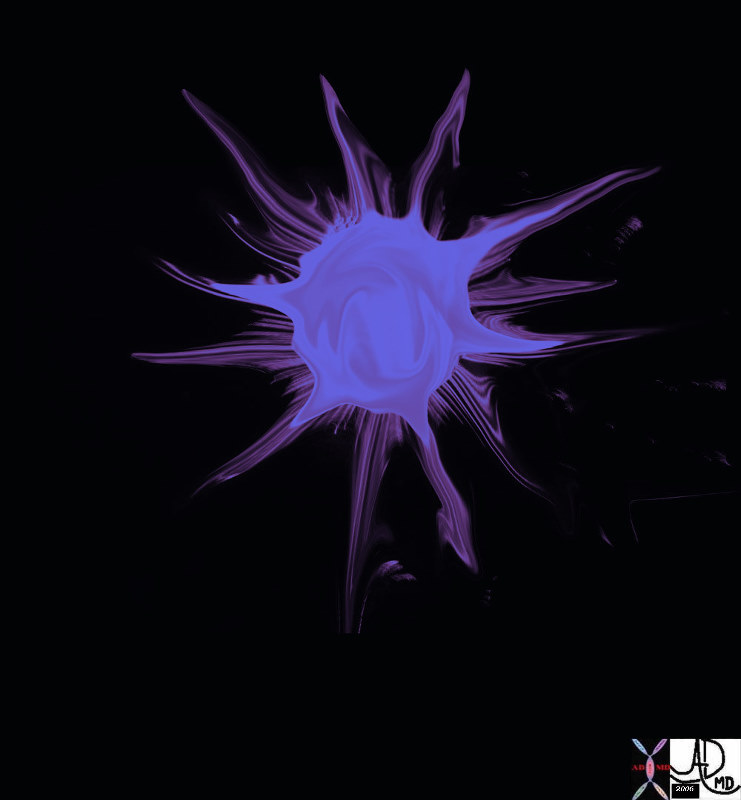
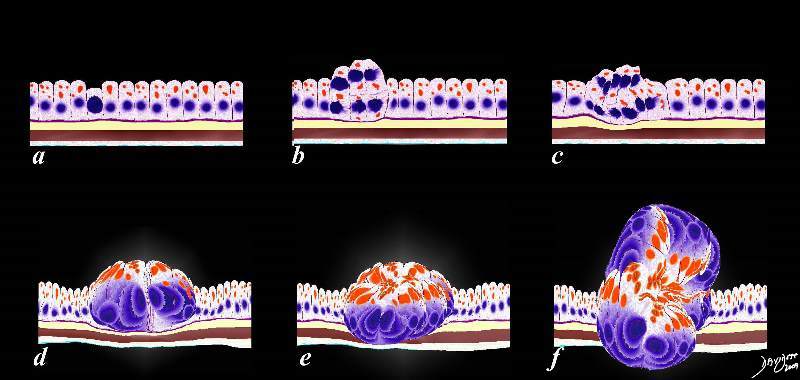


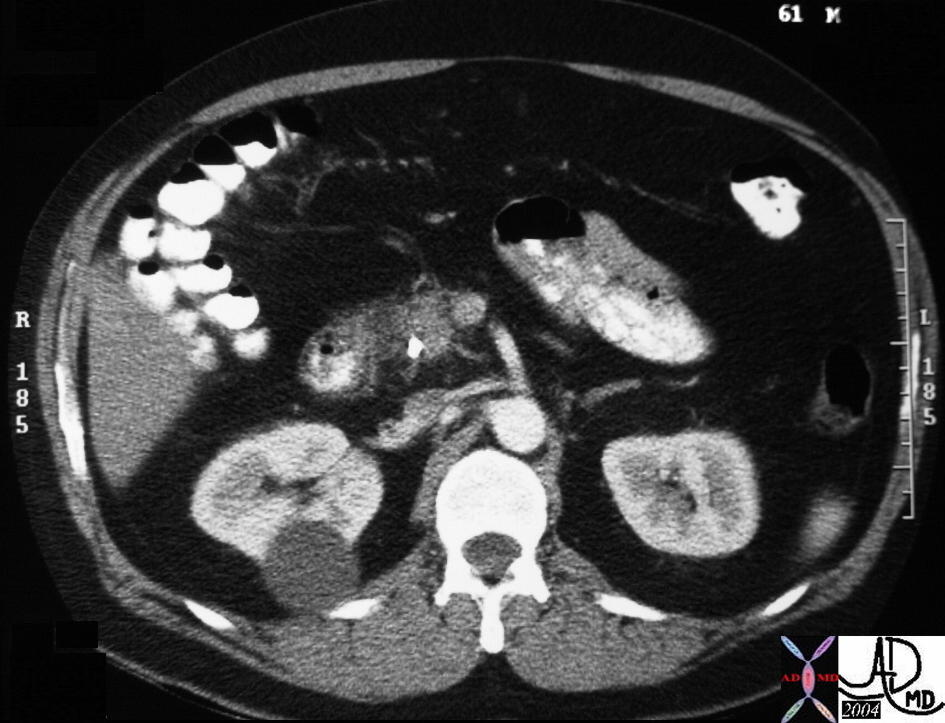
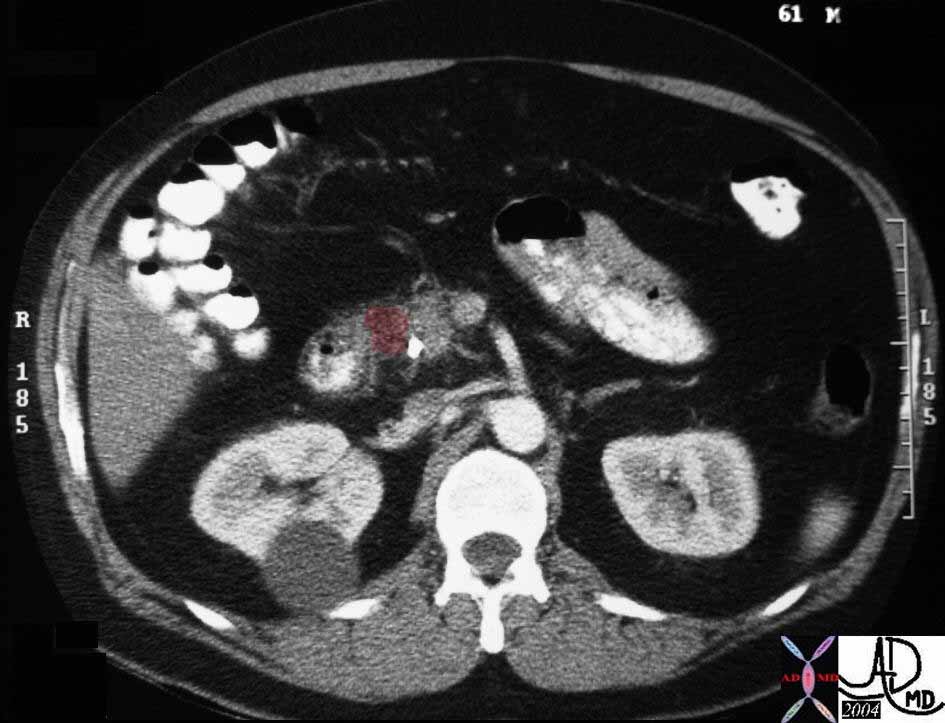
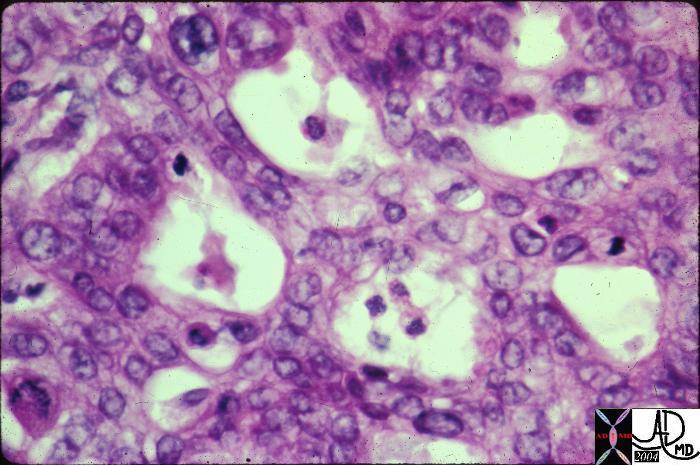
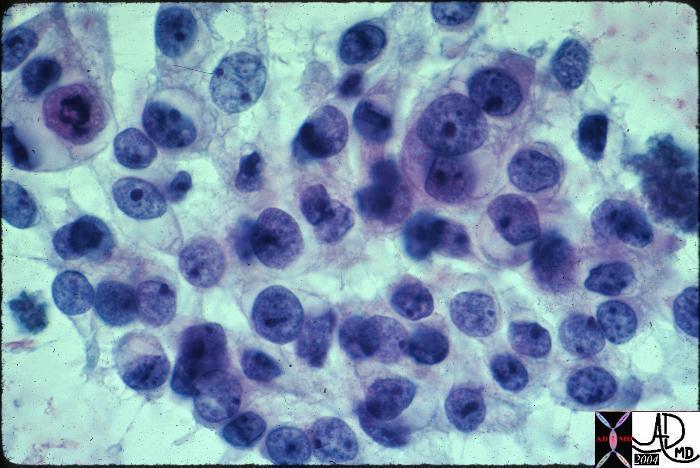
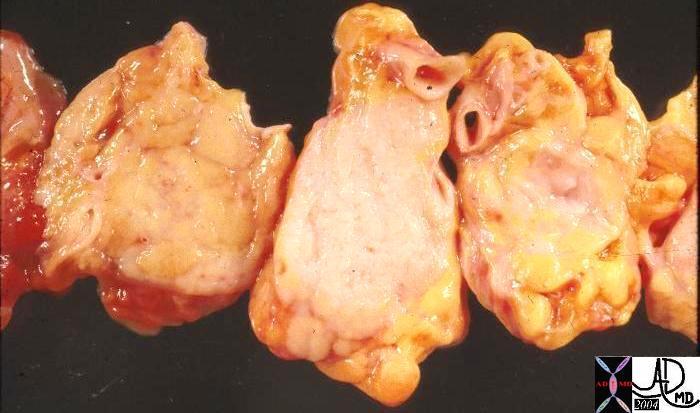
 The image from an MRCP shows the MRI version of the double duct. The separation of the two ducts suggests a large size of the mass. This patient had pancreatic carcinoma. 41371 Courtesy of Ashley Davidoff MD
The image from an MRCP shows the MRI version of the double duct. The separation of the two ducts suggests a large size of the mass. This patient had pancreatic carcinoma. 41371 Courtesy of Ashley Davidoff MD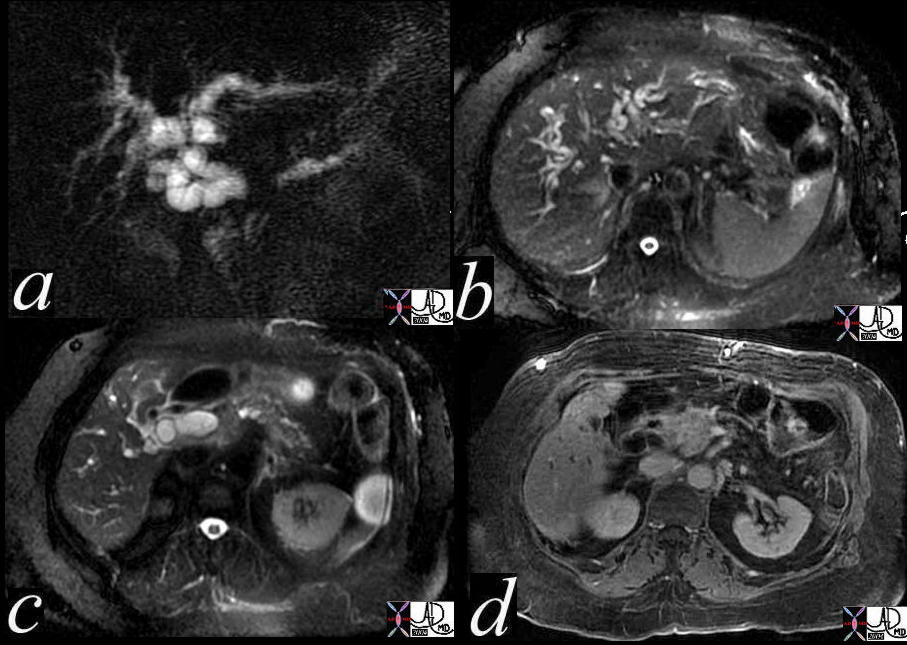
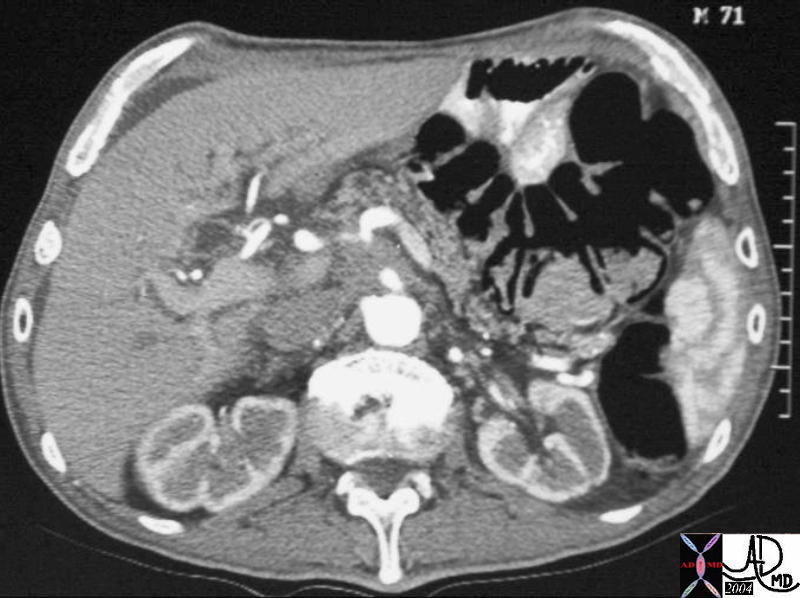
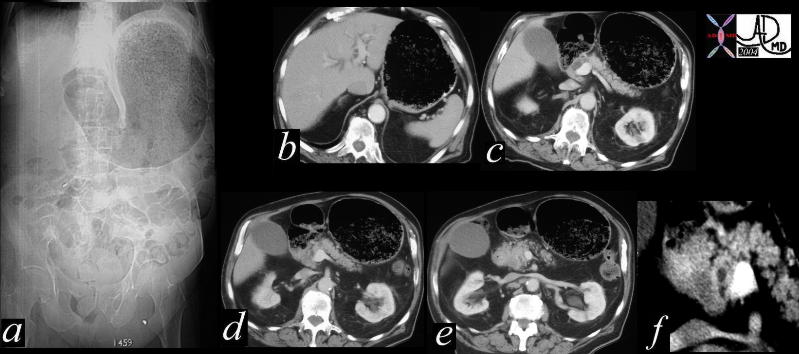
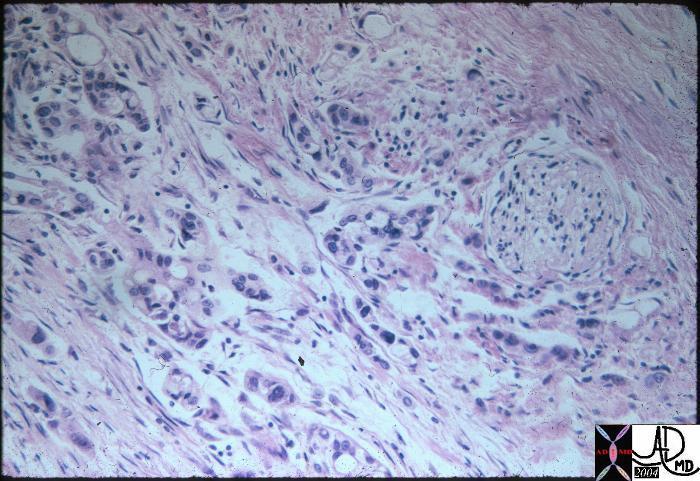
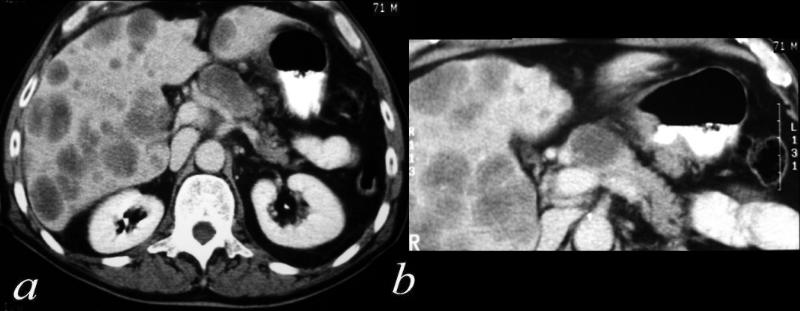
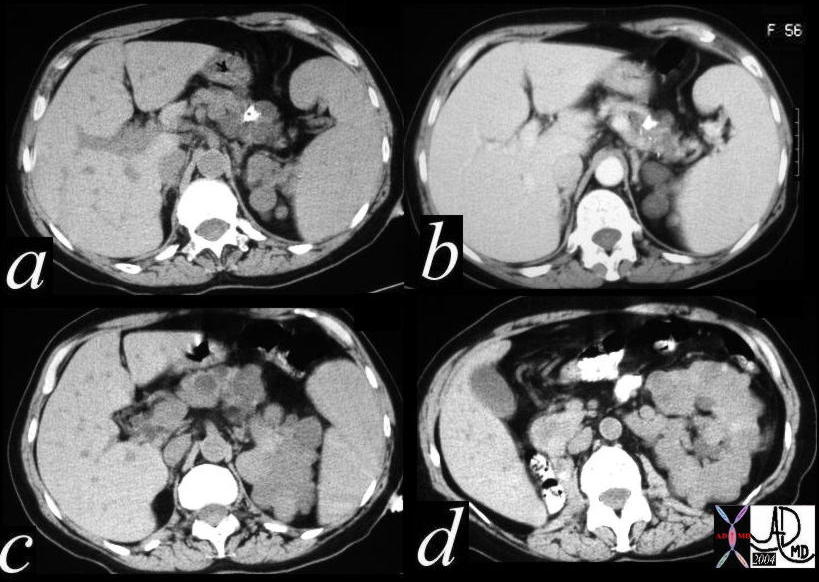
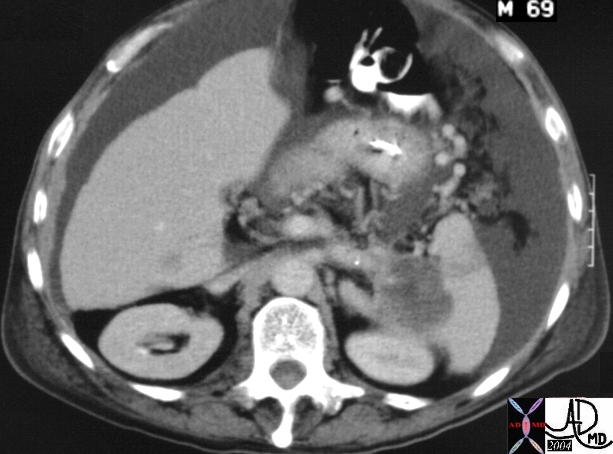
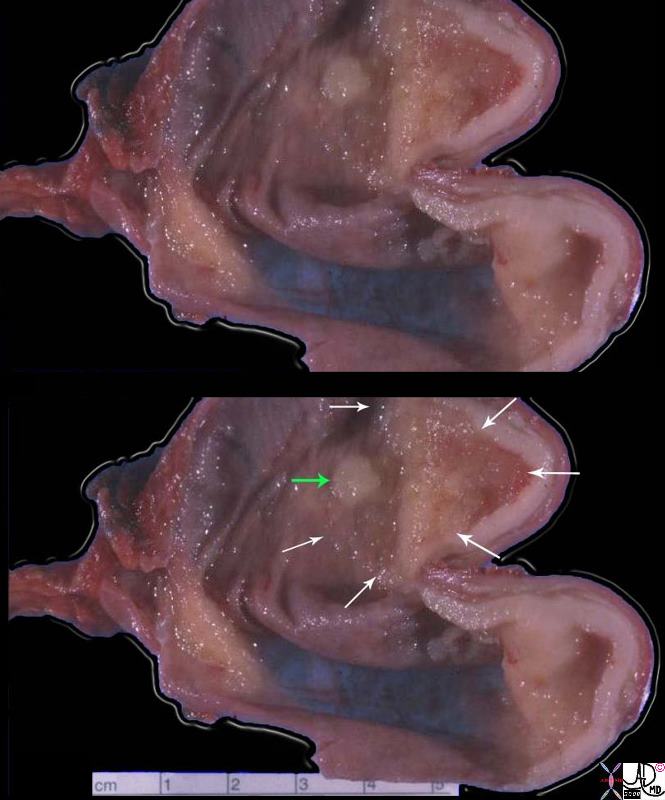 Carcinoma Thickening and Granular Appearance
Carcinoma Thickening and Granular Appearance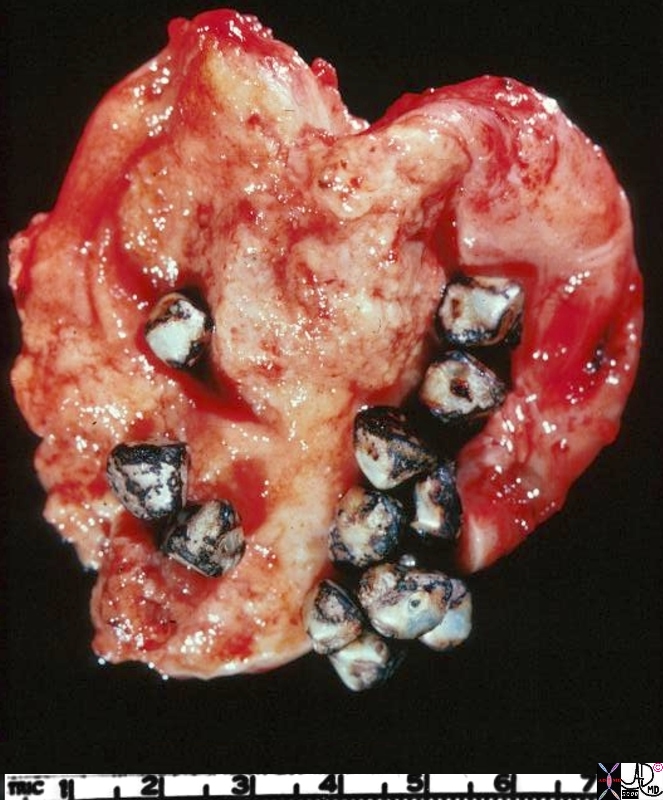 Carcinoma – Mass
Carcinoma – Mass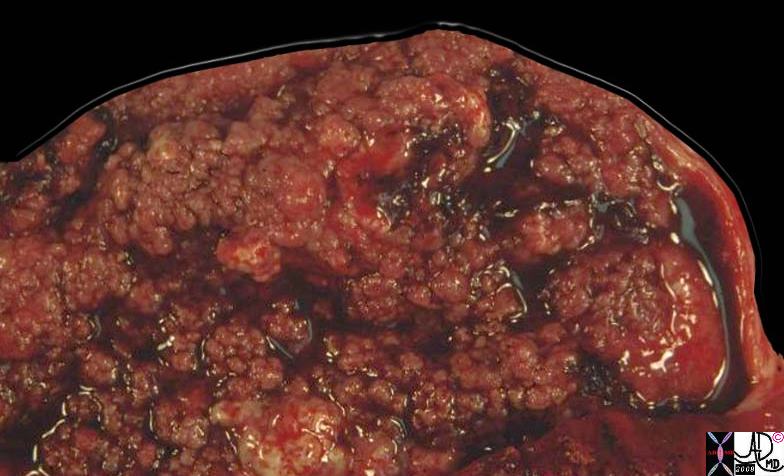
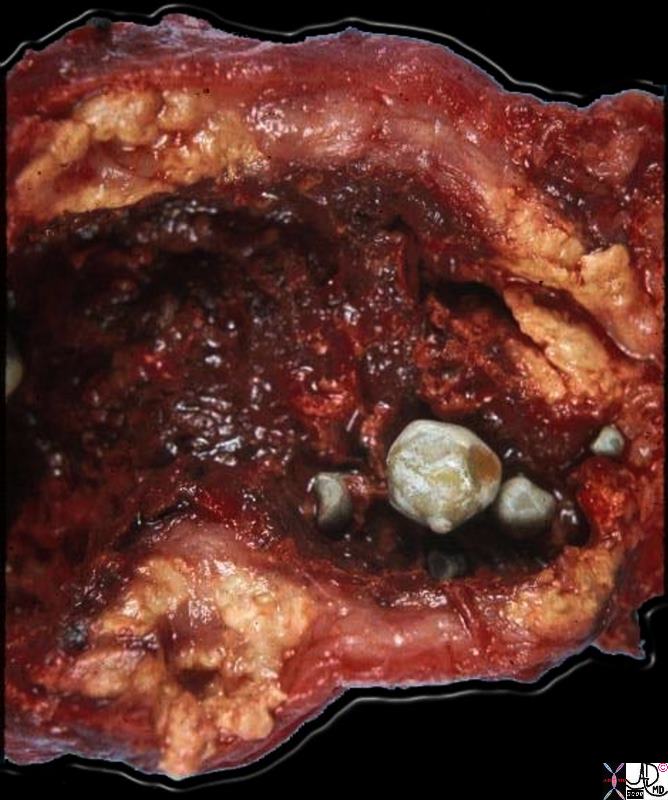
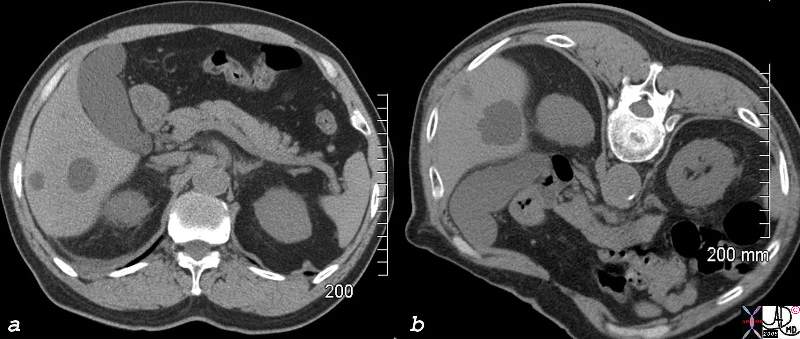
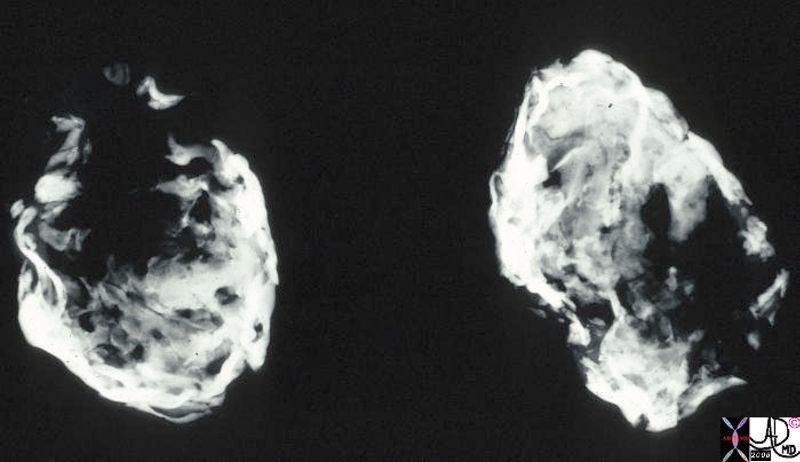
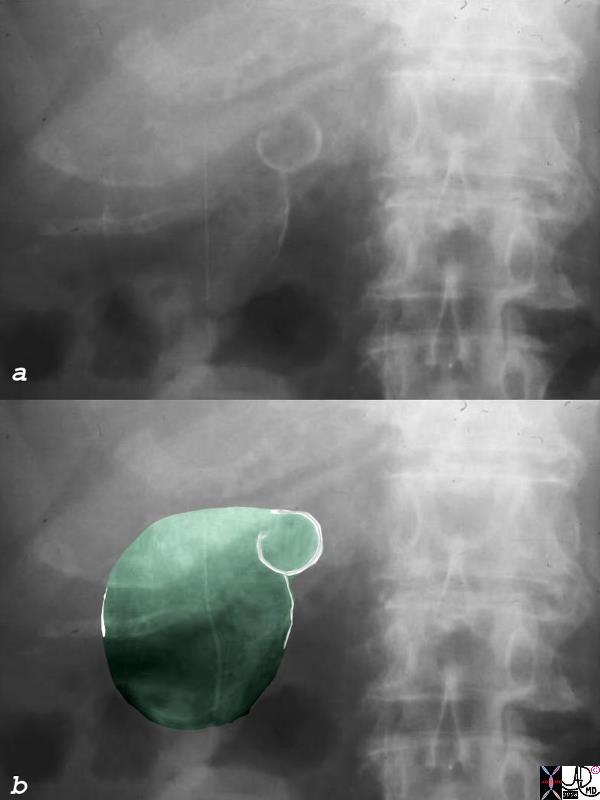

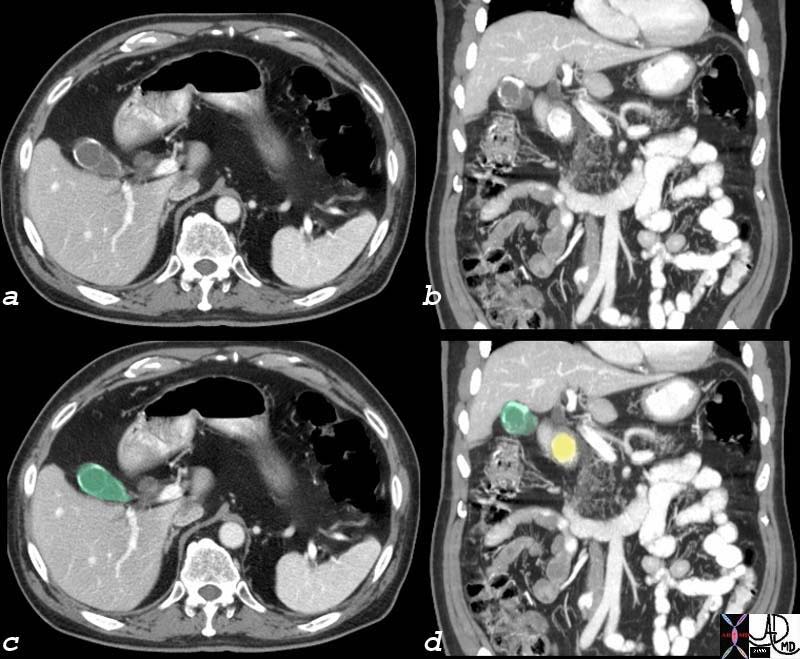

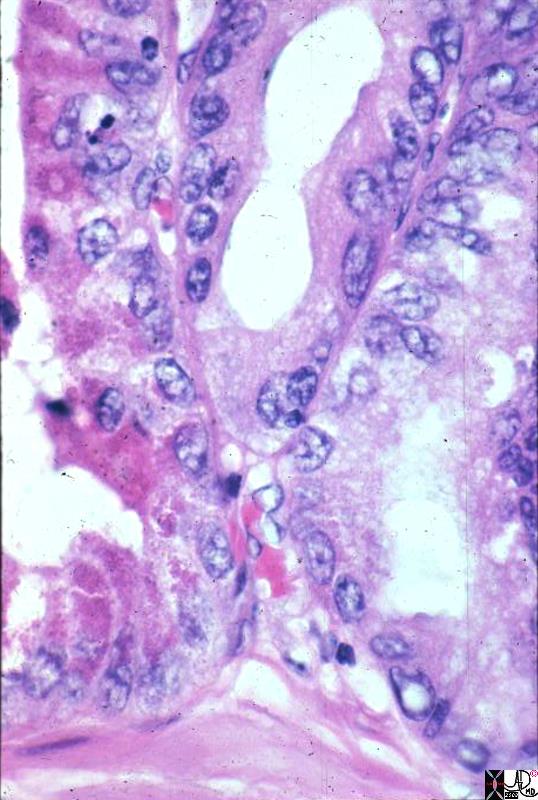
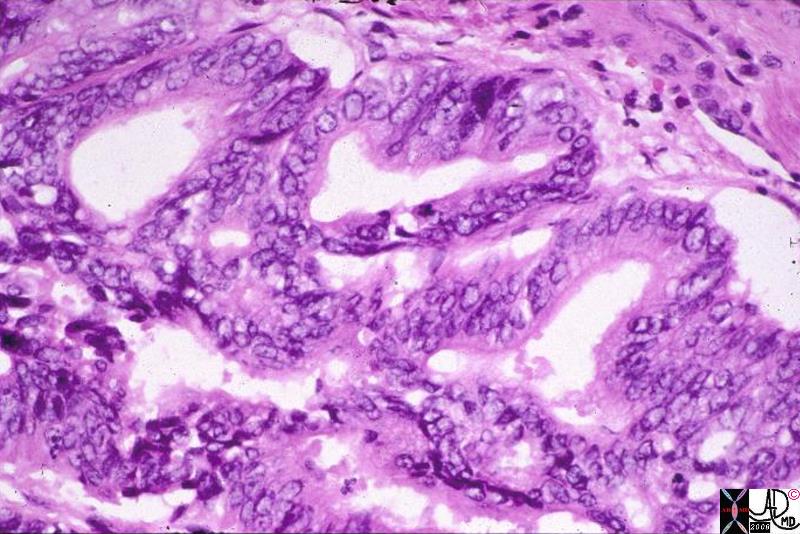 Carcinoma – Back to Back Gland Arrangement
Carcinoma – Back to Back Gland Arrangement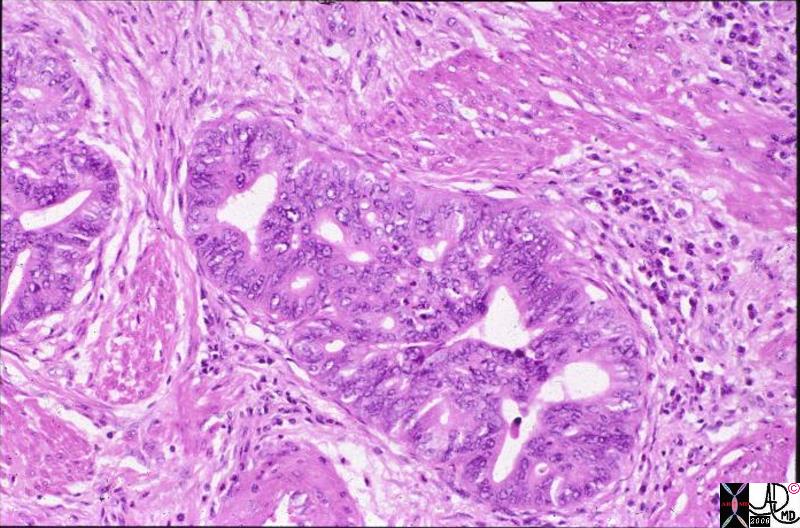 Carcinoma – Back to Back Gland Arrangement and Irregular Nuclii
Carcinoma – Back to Back Gland Arrangement and Irregular Nuclii Carcinoma – Papillary Form
Carcinoma – Papillary Form Carcinoma – Lymph Nodes
Carcinoma – Lymph Nodes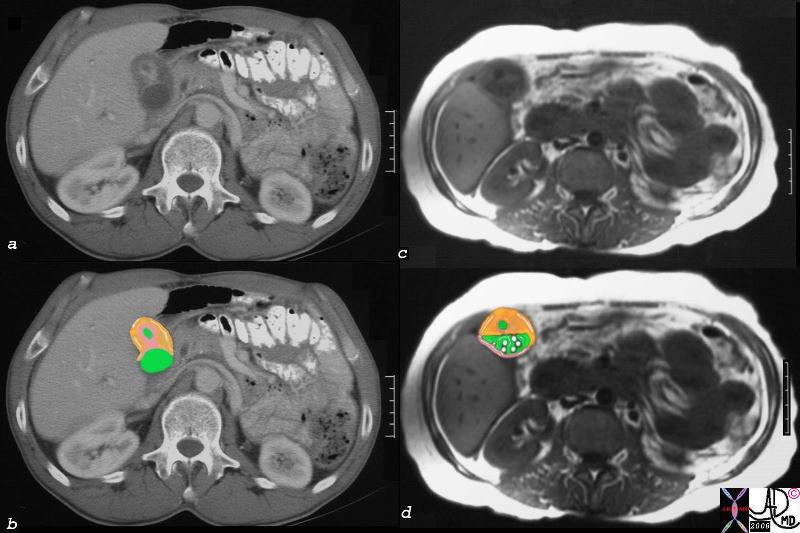 Carcinoma of the Fundus CT and MRI
Carcinoma of the Fundus CT and MRI
 Direct Invasion into the Liver and Bile Duct Obstruction
Direct Invasion into the Liver and Bile Duct Obstruction Dystrophic Calcification – Carcinoma of the Gallbladder Extending into the Gallbladder Fossa
Dystrophic Calcification – Carcinoma of the Gallbladder Extending into the Gallbladder Fossa Local Invasion and Separate Liver Mestastasis
Local Invasion and Separate Liver Mestastasis
 Luminal Invasion
Luminal Invasion Gallbladder Carcinoma with Perforation and Abscess Formation
Gallbladder Carcinoma with Perforation and Abscess Formation
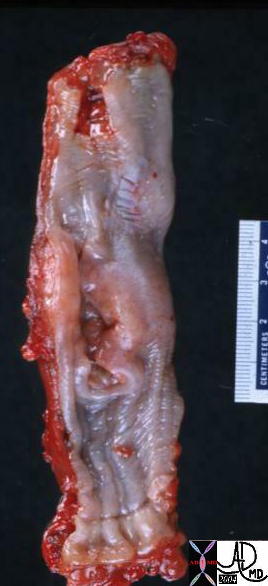
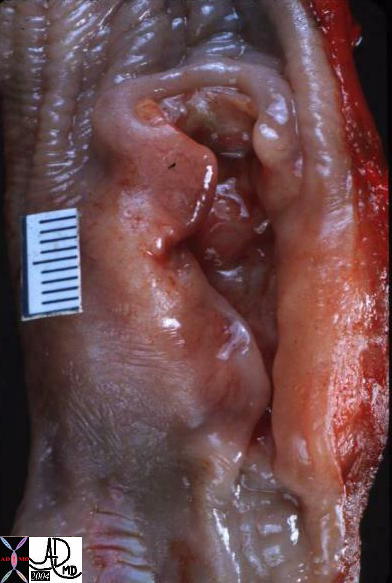 Ulcerating Squamous Cell Carcinoma of the Esophagus
Ulcerating Squamous Cell Carcinoma of the Esophagus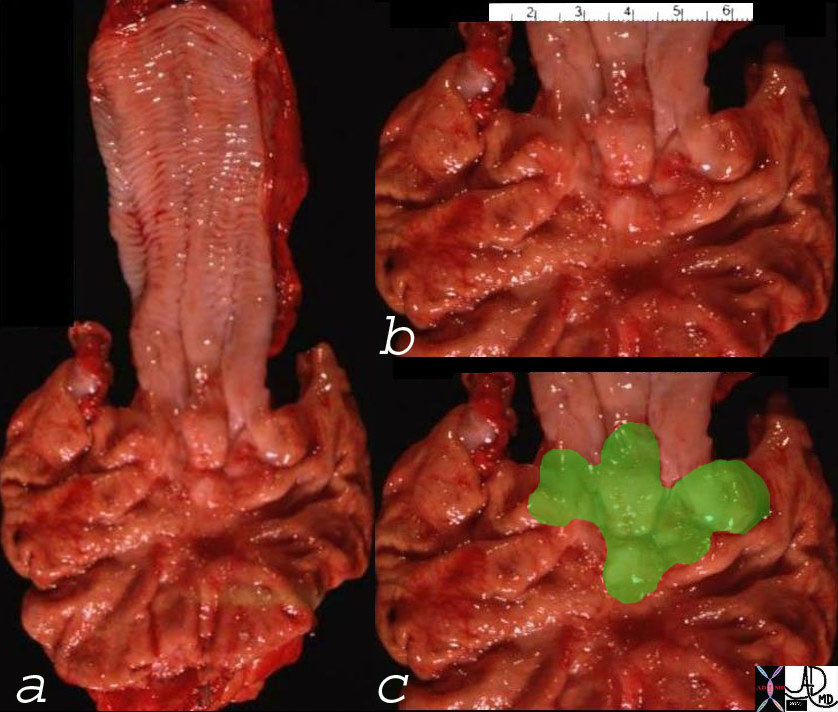 Ill Defined GE Junction Tumor – Adenocarcinoma
Ill Defined GE Junction Tumor – Adenocarcinoma Carcinoma of the Esophagus
Carcinoma of the Esophagus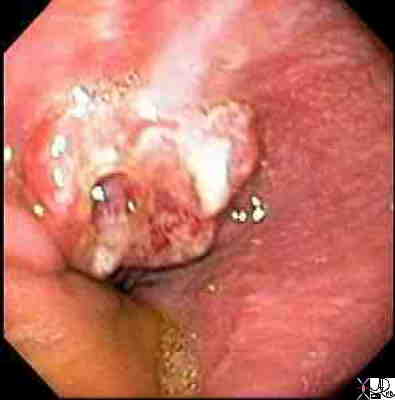 Carcinoma of the Esophagus
Carcinoma of the Esophagus Irregular Distal Esophagus with Stricture – Adenocarcinoma of the GE junction
Irregular Distal Esophagus with Stricture – Adenocarcinoma of the GE junction Long Relatively Smooth Malignant Stricture
Long Relatively Smooth Malignant Stricture
 Staging by Non Invasive Method
Staging by Non Invasive Method Stent in the Distal Esophagus
Stent in the Distal Esophagus
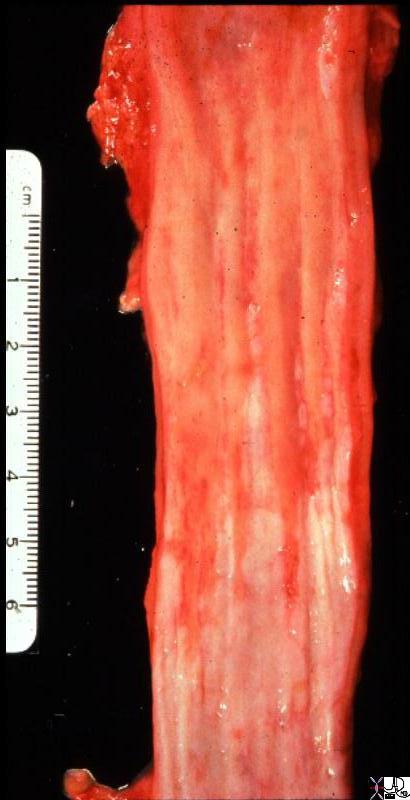
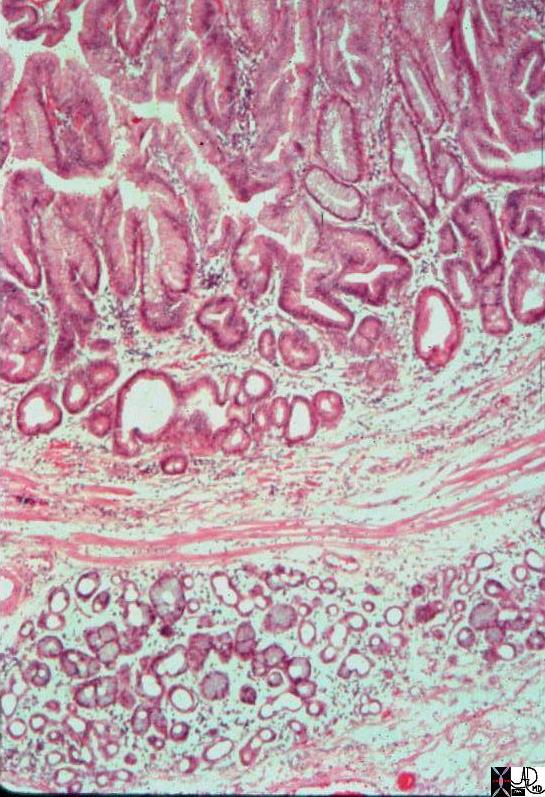 Barretts Esophagus
Barretts Esophagus Barrett’s Esophagus
Barrett’s Esophagus Barret’s Esophagus by Endoscopy No Specific Findings
Barret’s Esophagus by Endoscopy No Specific Findings
 Ulcerating Colon Carcinoma
Ulcerating Colon Carcinoma Mucosal disease -a benign polyp in its early stages
Mucosal disease -a benign polyp in its early stages Mucosal disease –beginning of a neoplasm
Mucosal disease –beginning of a neoplasm 5mm benign polyp
5mm benign polyp
 Apple core lesion of the sigmoid colon
Apple core lesion of the sigmoid colon Napkin Ring Circunferential Tumor -aka Apple Core
Napkin Ring Circunferential Tumor -aka Apple Core Synchronous Tumors Lying Side By Side in the Cecum
Synchronous Tumors Lying Side By Side in the Cecum Familial Polyposis Syndrome with Carcinoma
Familial Polyposis Syndrome with Carcinoma
 Colon Carcinoma in a Background of Chronic Ulcerative Colitis
Colon Carcinoma in a Background of Chronic Ulcerative Colitis This polyp is greater than 2cms and was malignant on histopathologyical examination
This polyp is greater than 2cms and was malignant on histopathologyical examination
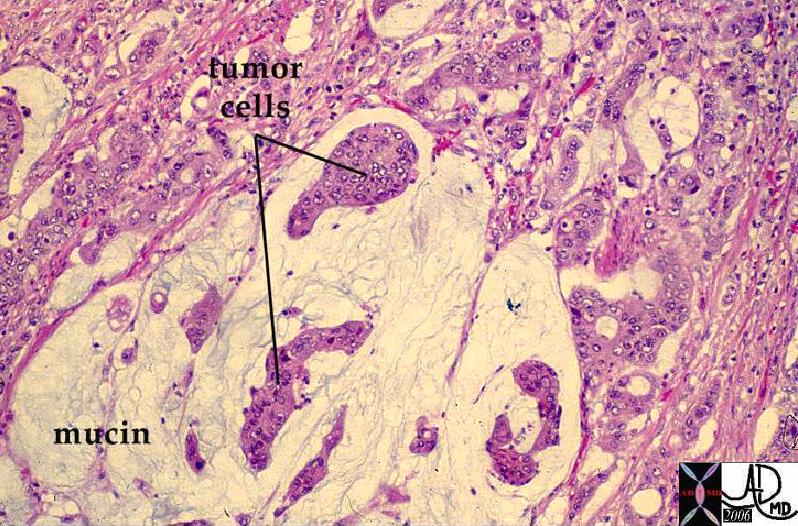 Normal Colonic Mucosa (left) and Mucin Secreting Colon Carcinoma (right)
Normal Colonic Mucosa (left) and Mucin Secreting Colon Carcinoma (right) Colon Carcinoma – Cytopathology
Colon Carcinoma – Cytopathology Carcinoma of the Colon with Metatstasis to the Liver
Carcinoma of the Colon with Metatstasis to the Liver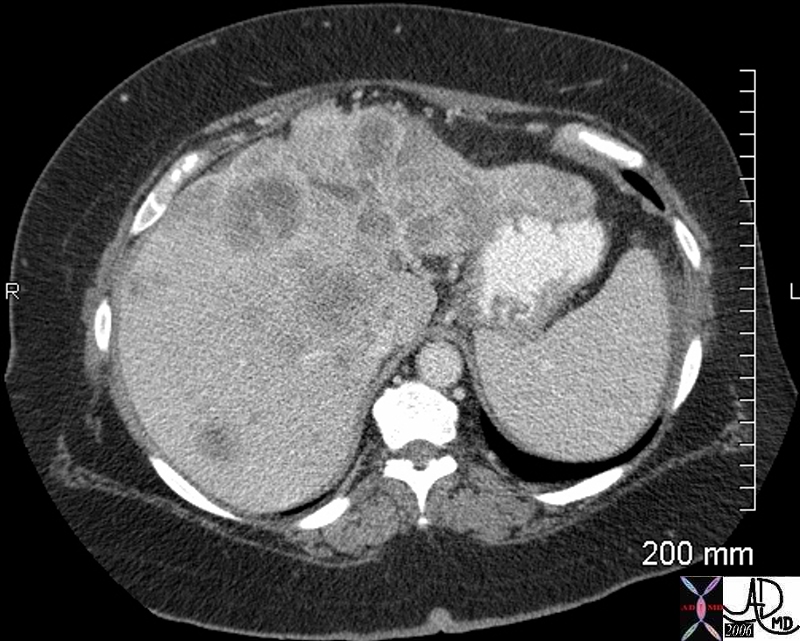
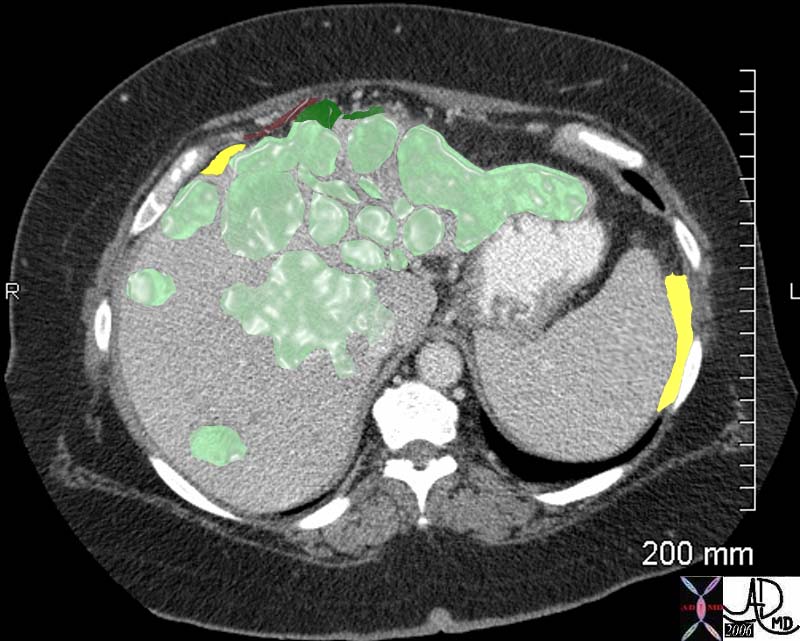 Colon Carcinoma with Multiple Metastases to the Liver and Involvement of the Capsule
Colon Carcinoma with Multiple Metastases to the Liver and Involvement of the Capsule Sigmoid Carcinoma with Metastases to the Liver and Lung (green)
Sigmoid Carcinoma with Metastases to the Liver and Lung (green)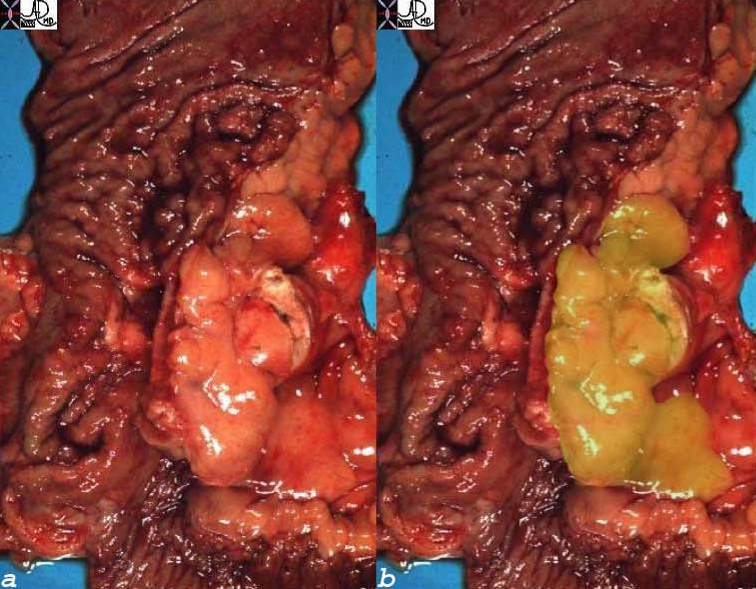 Regional Lymph Node Enlarged
Regional Lymph Node Enlarged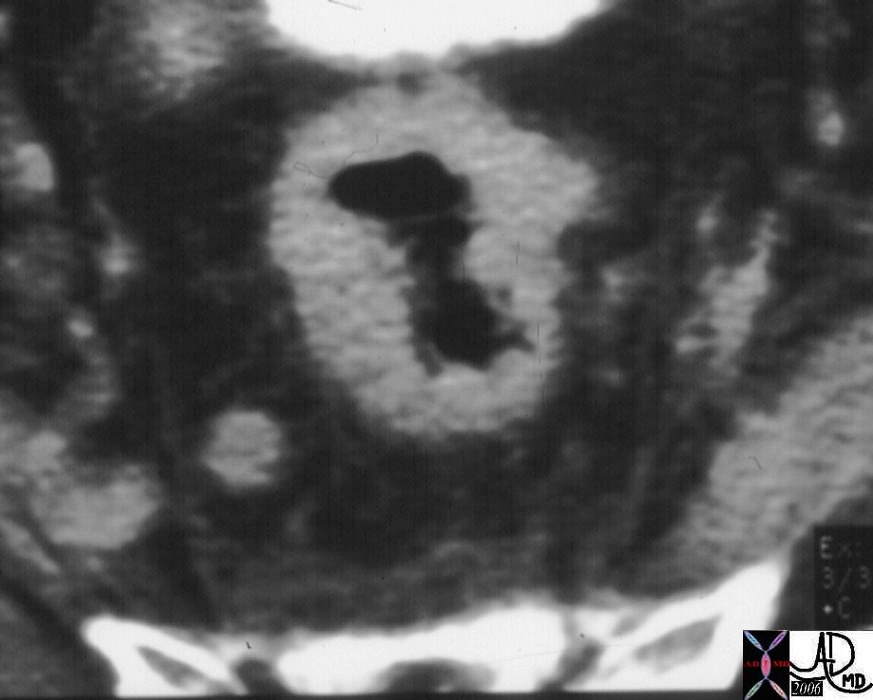
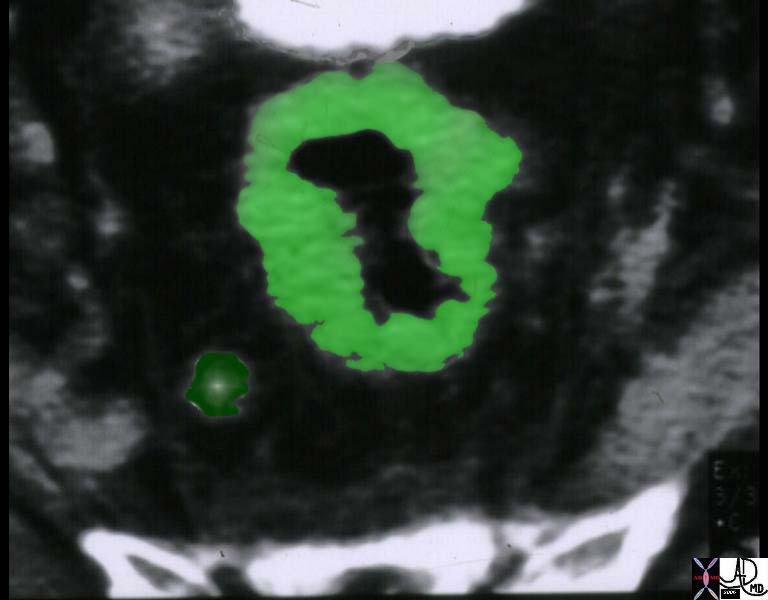 Rectal Carcinoma with a Perirectal Node
Rectal Carcinoma with a Perirectal Node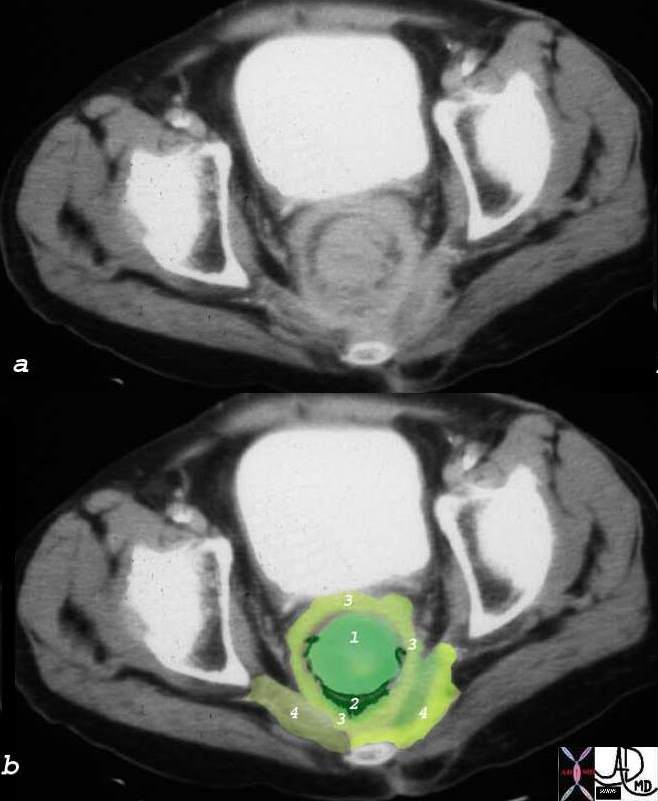 Obsructing Rectal Carcinoma with Extension into Levator Ani and Pelvic Wall
Obsructing Rectal Carcinoma with Extension into Levator Ani and Pelvic Wall Colonic Distension No Rectal Air
Colonic Distension No Rectal Air
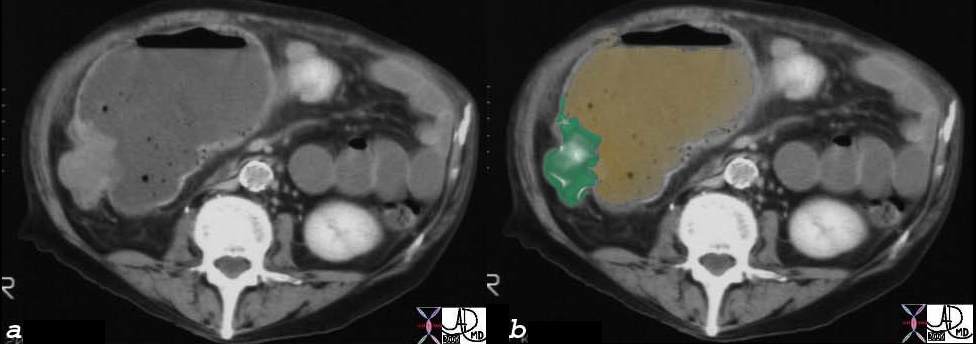 Obstructing Colon Carcinoma with Upstream Dilatation
Obstructing Colon Carcinoma with Upstream Dilatation Cecal Carcinoma with Peritoneal Involvement presents with RLQ Pain
Cecal Carcinoma with Peritoneal Involvement presents with RLQ Pain Endoscopic Ultrasound Showing a 1.5cms Rectal Tumor That Has Extended into the Submucosa and Muscularis In the TNM classification sytem it would be a T3 lesion
Endoscopic Ultrasound Showing a 1.5cms Rectal Tumor That Has Extended into the Submucosa and Muscularis In the TNM classification sytem it would be a T3 lesion
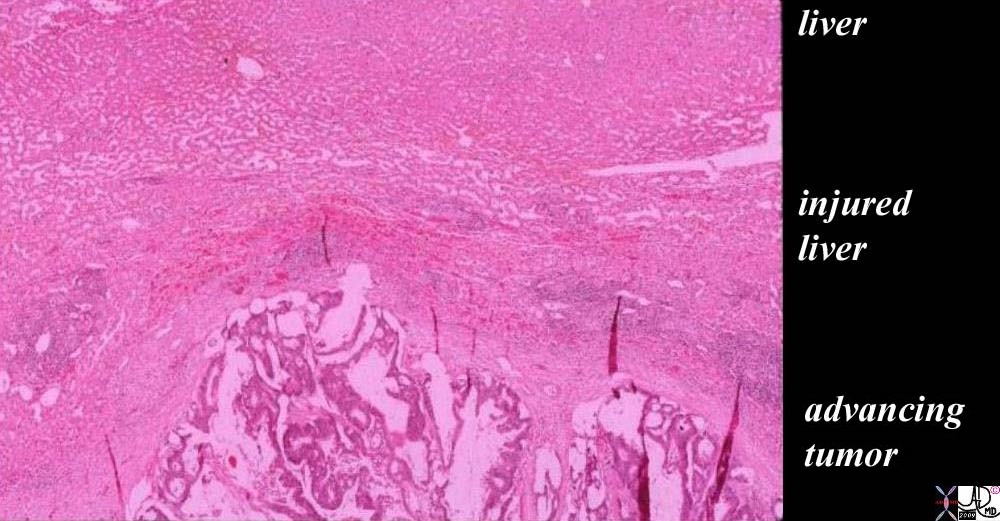
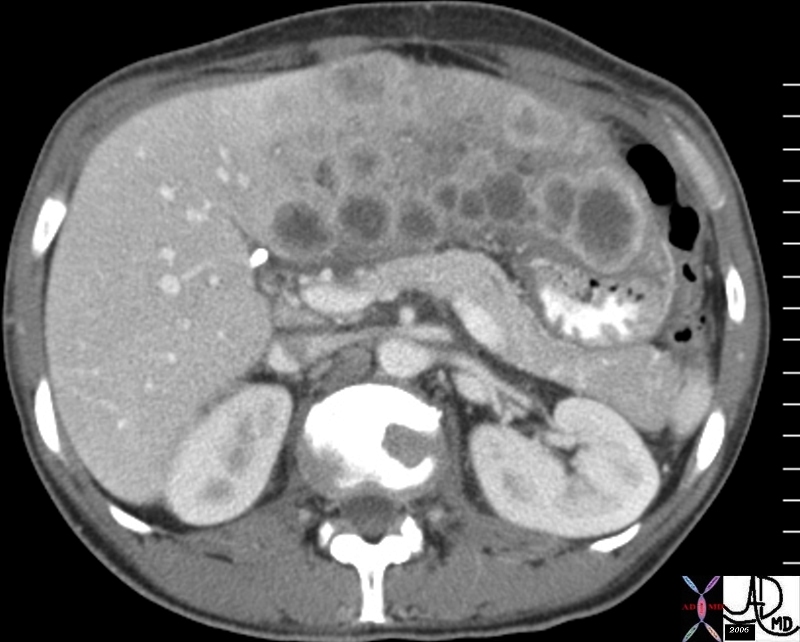
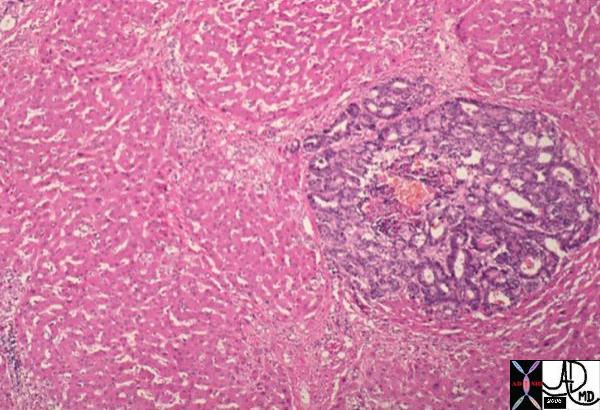
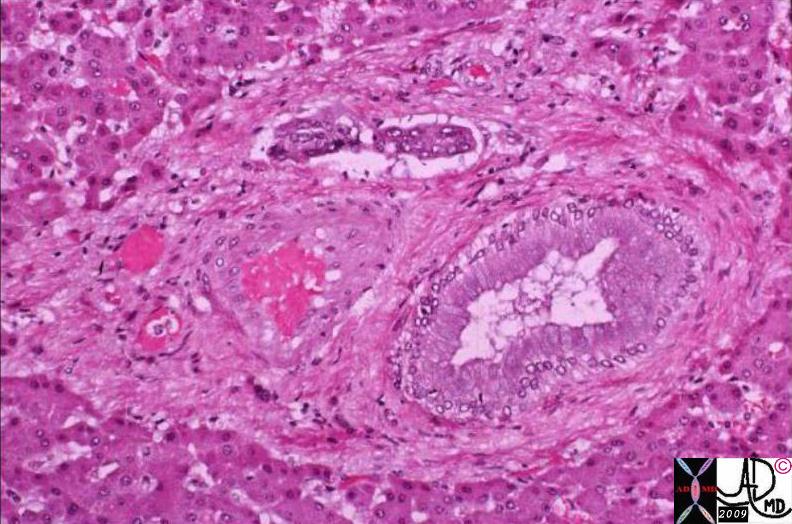
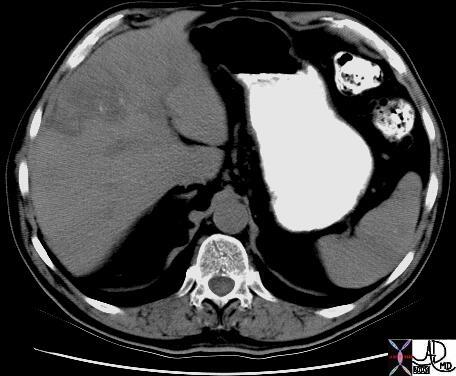
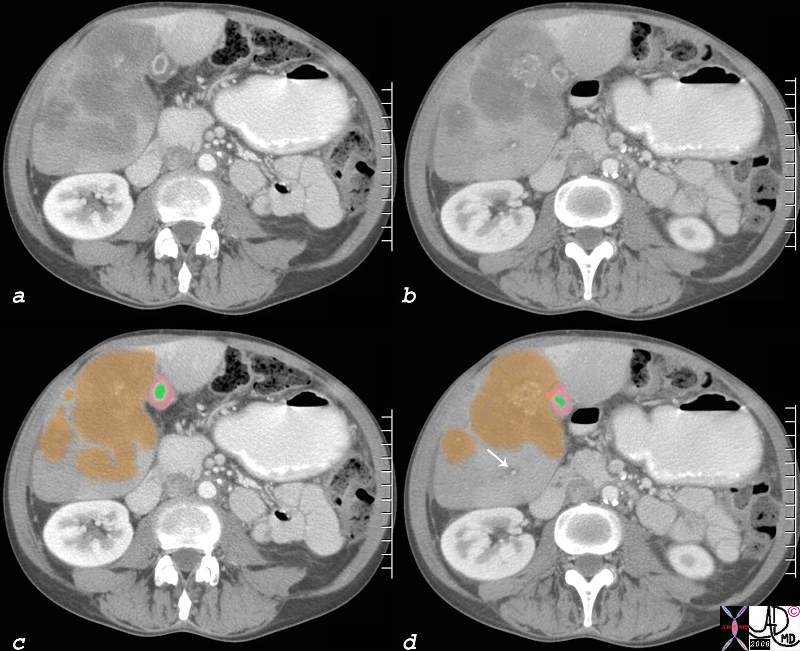




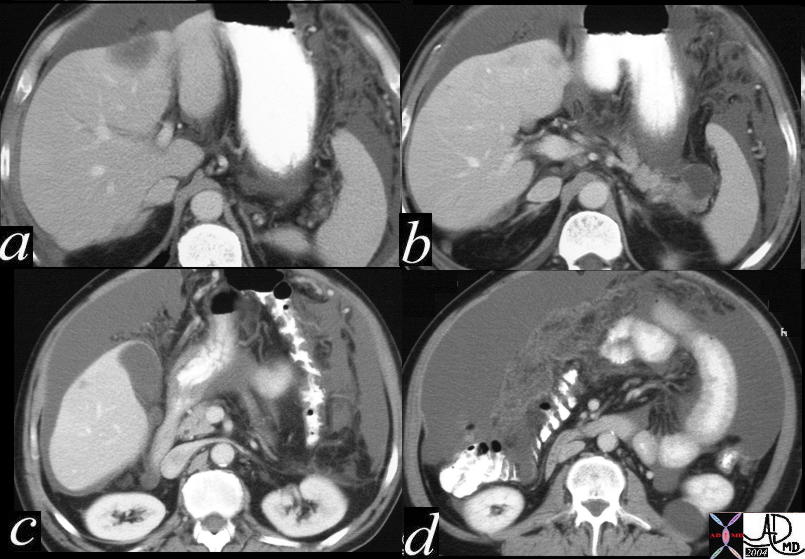
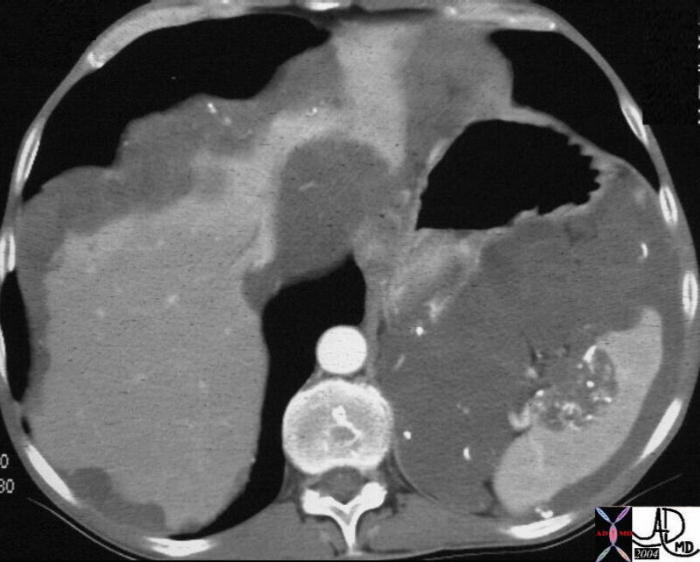

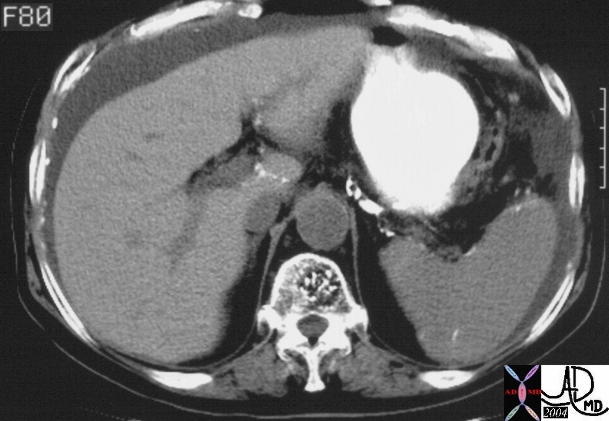
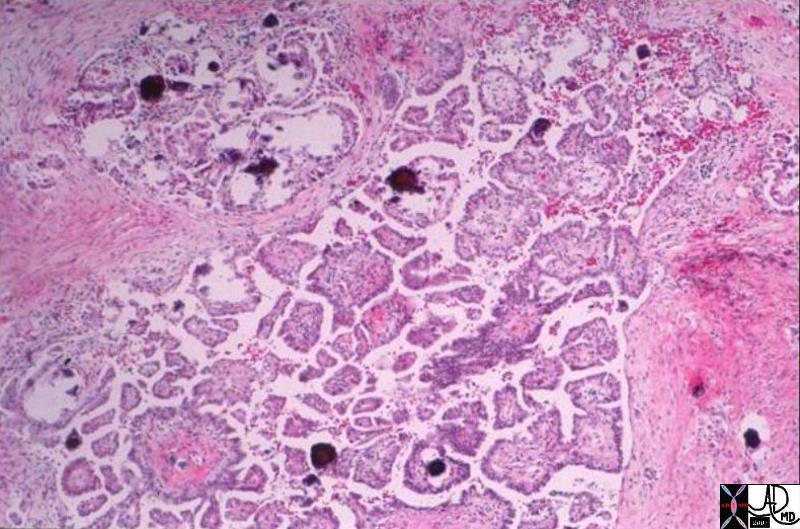
 0886
0886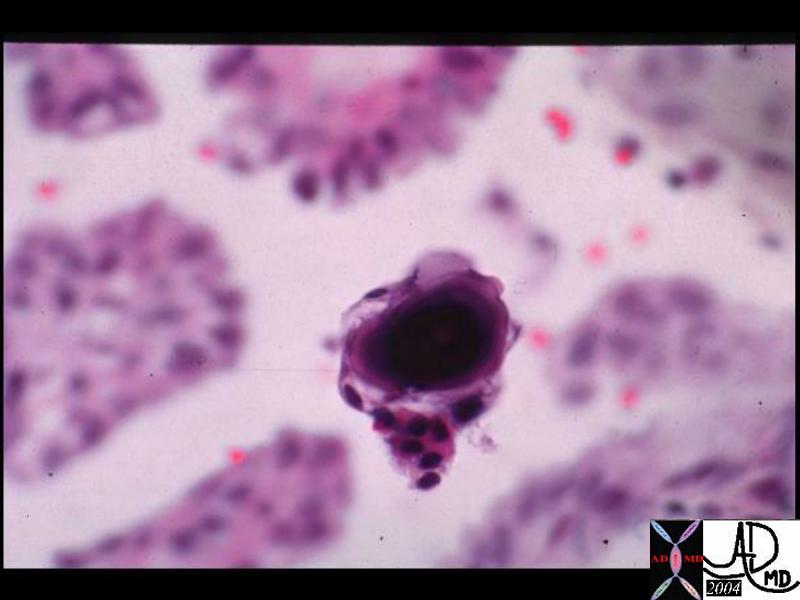
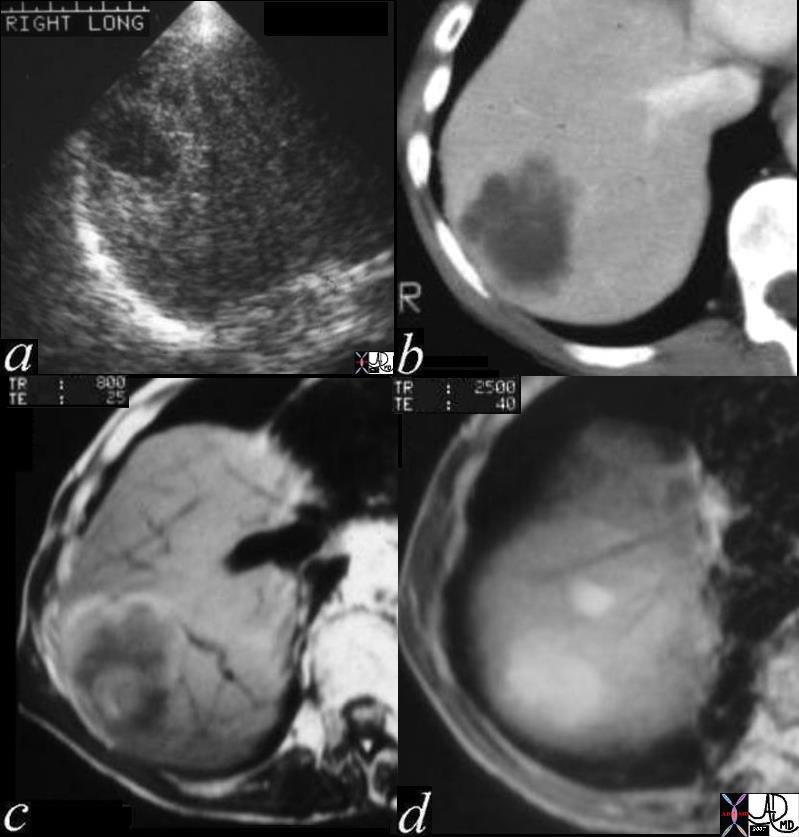
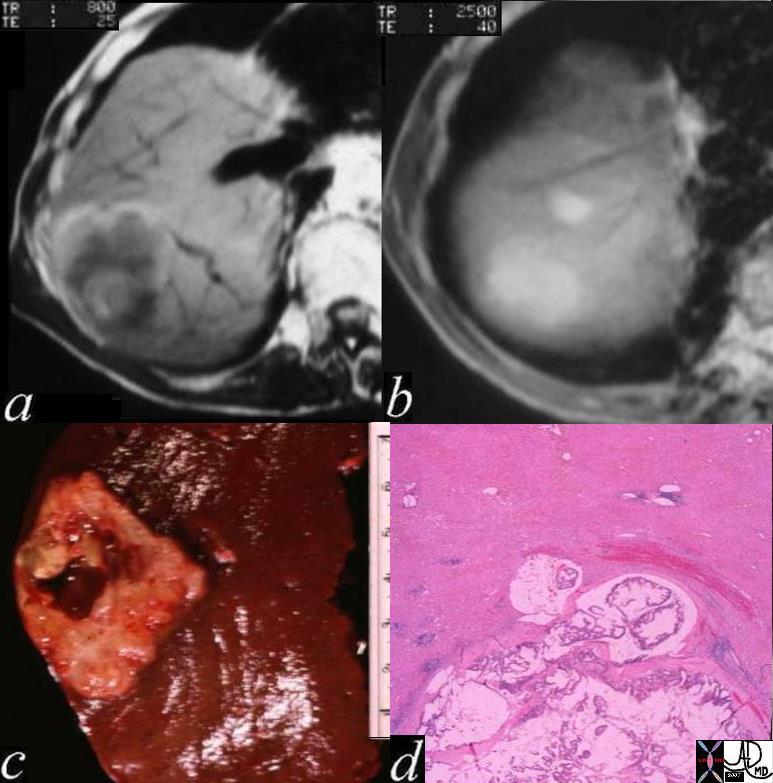
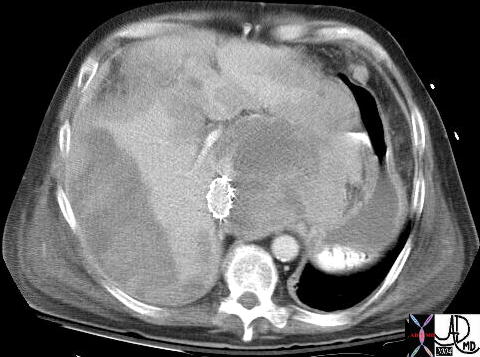
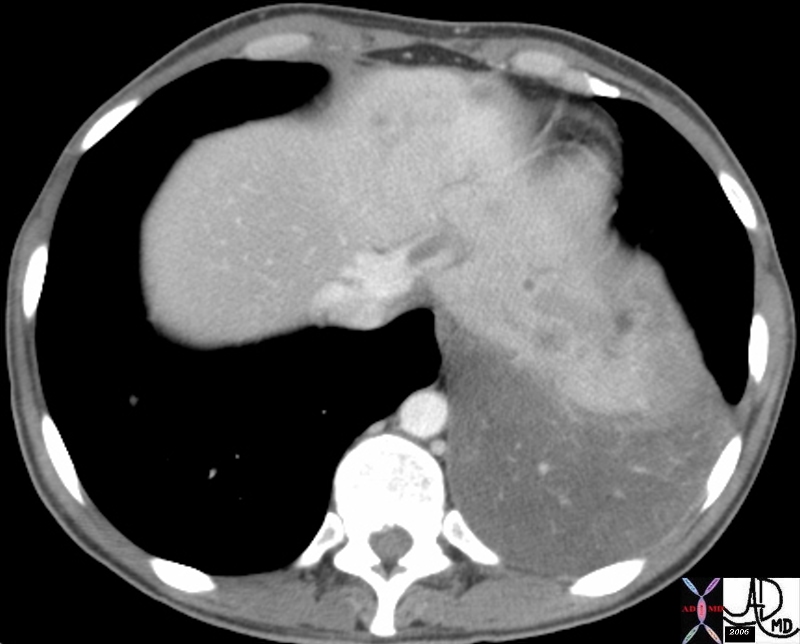
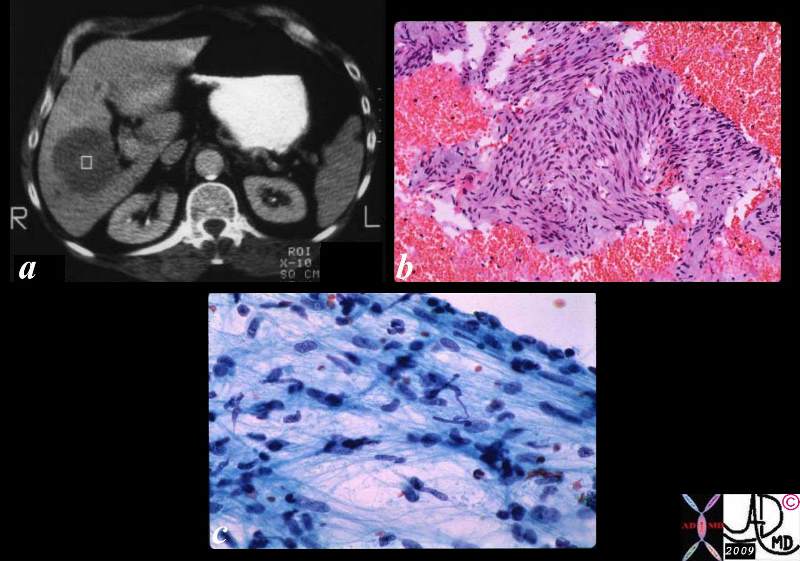
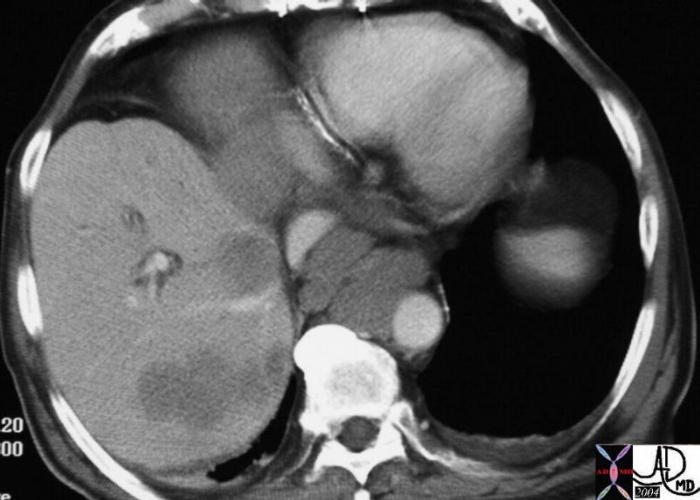
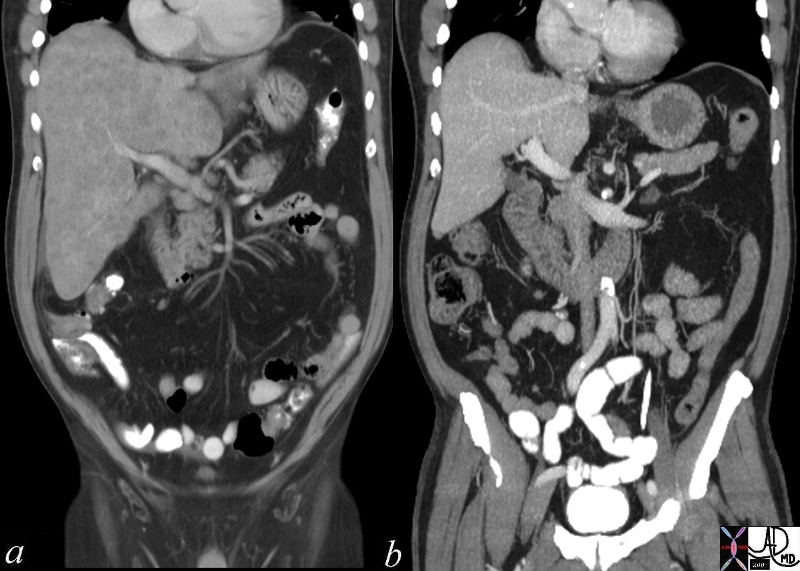
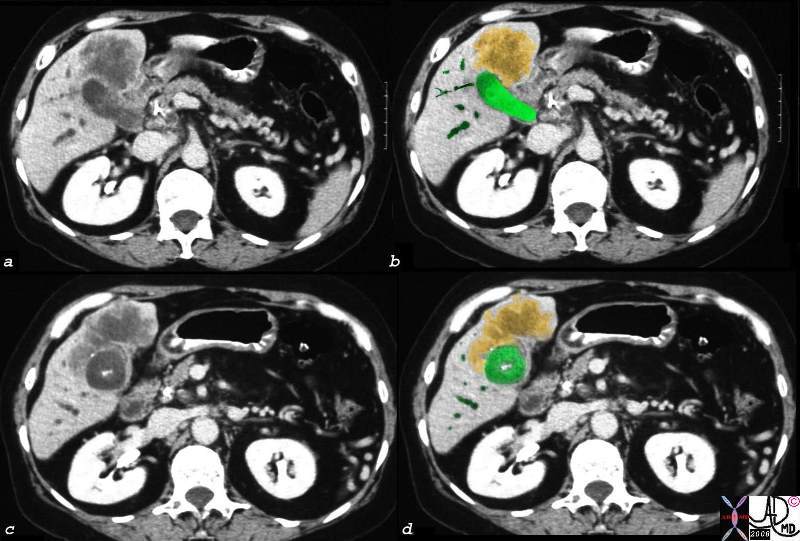
 Liver Cancer Occupying Space Distorting Structure and Function
Liver Cancer Occupying Space Distorting Structure and Function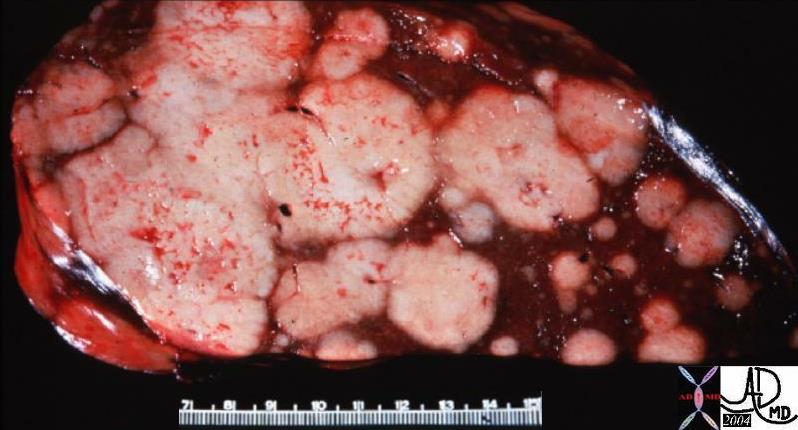 Space Occupation in the Liver
Space Occupation in the Liver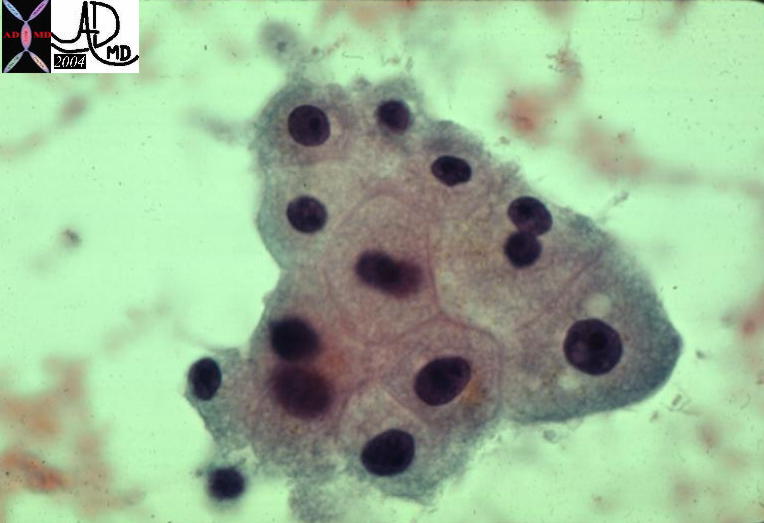
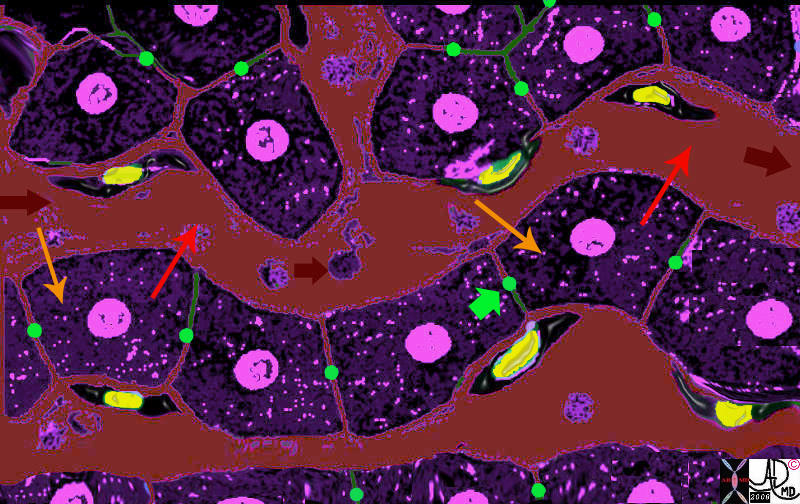

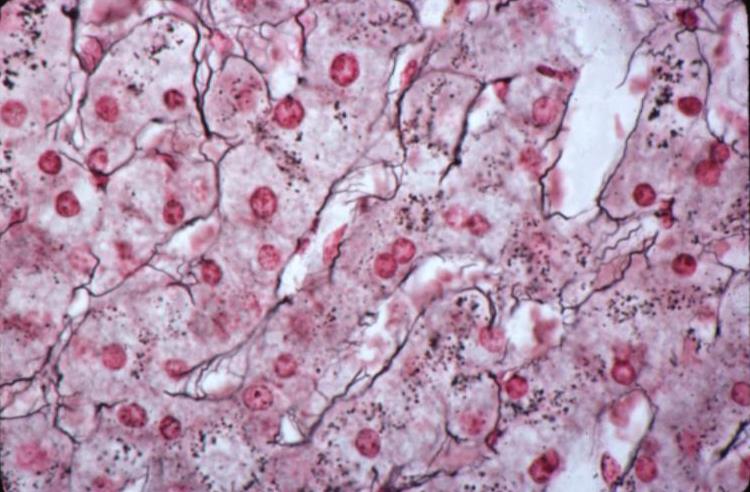




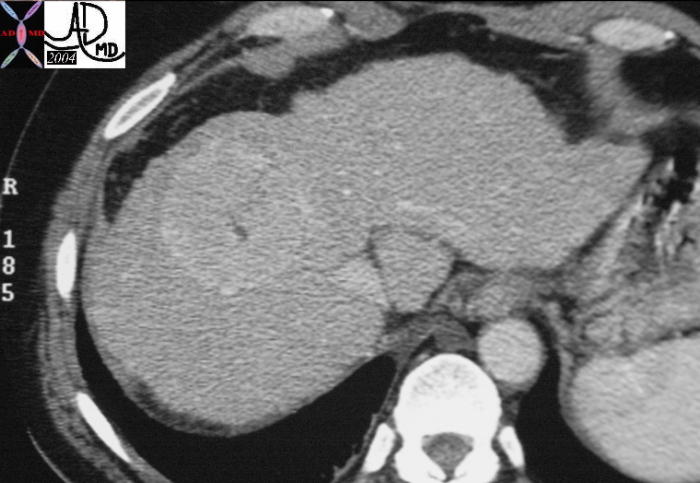
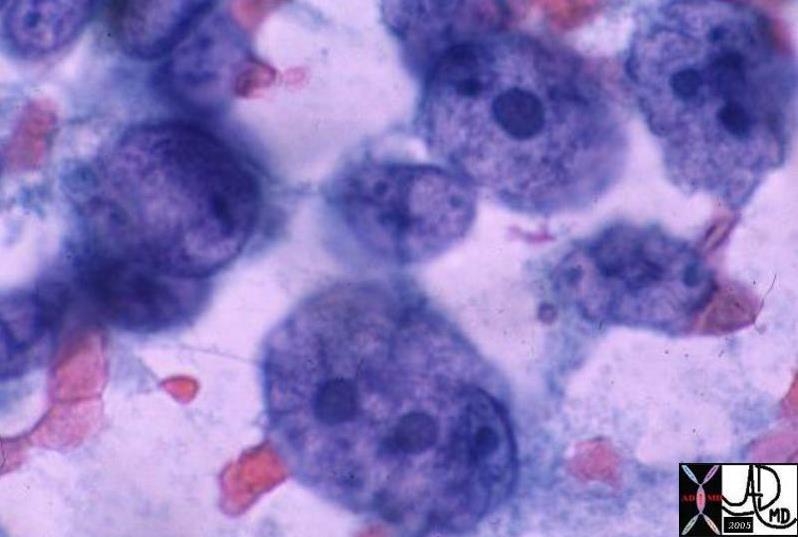 03539
03539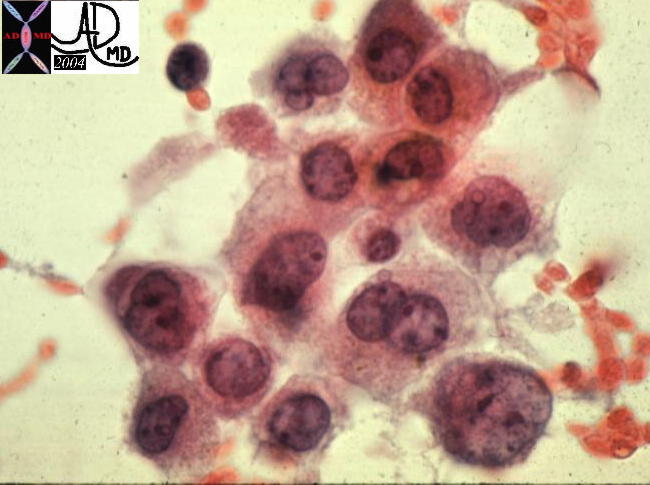 13447
13447 48369c03
48369c03

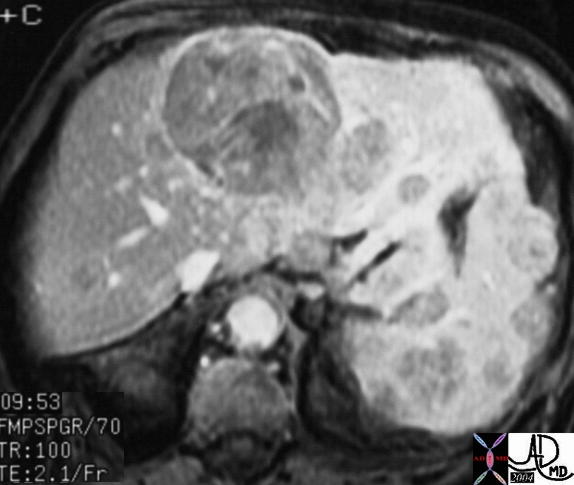
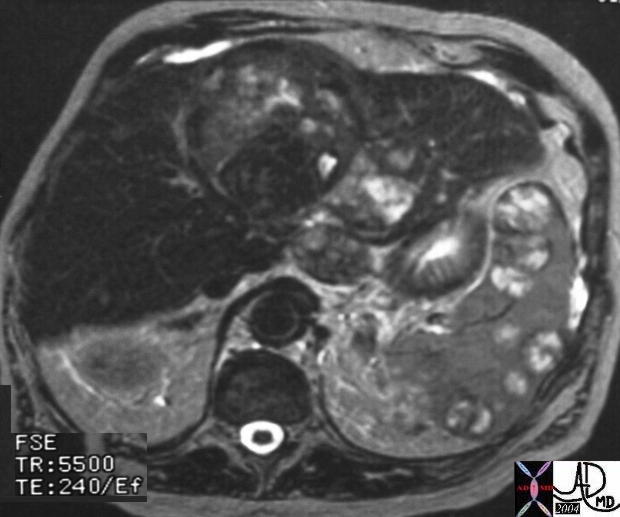
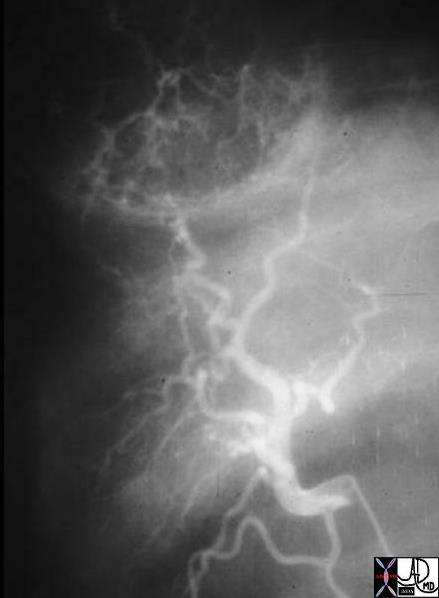
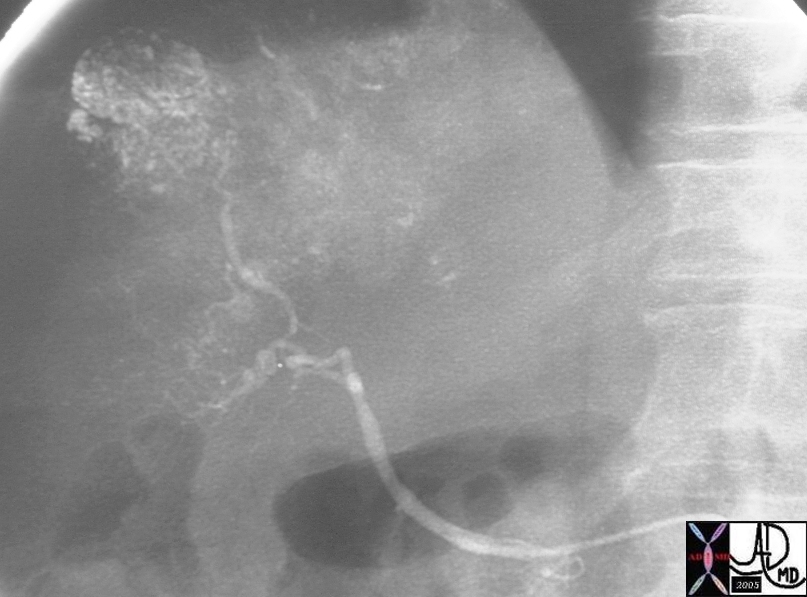
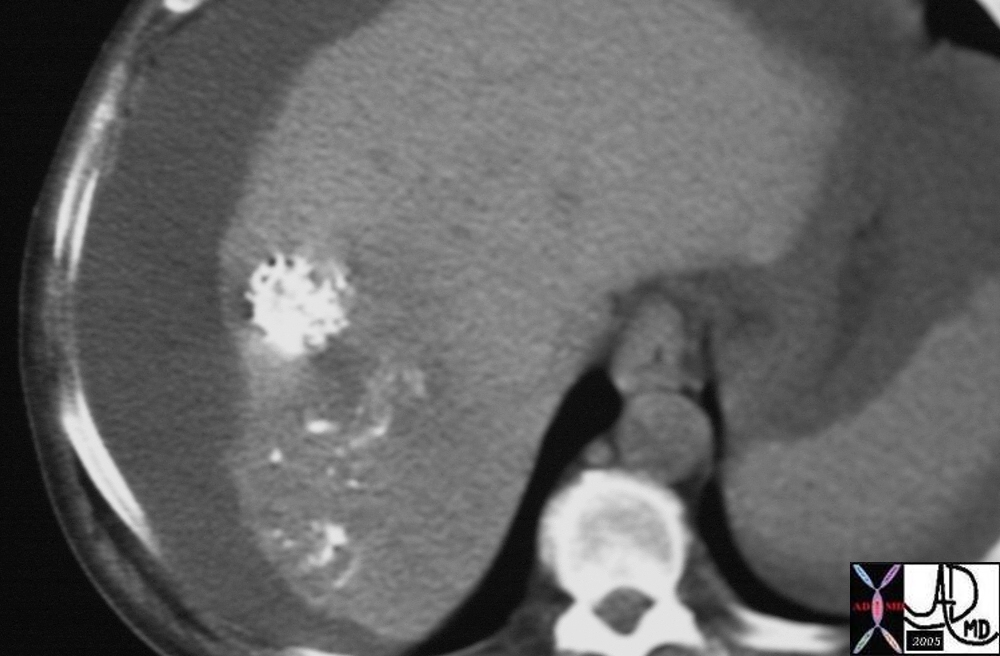
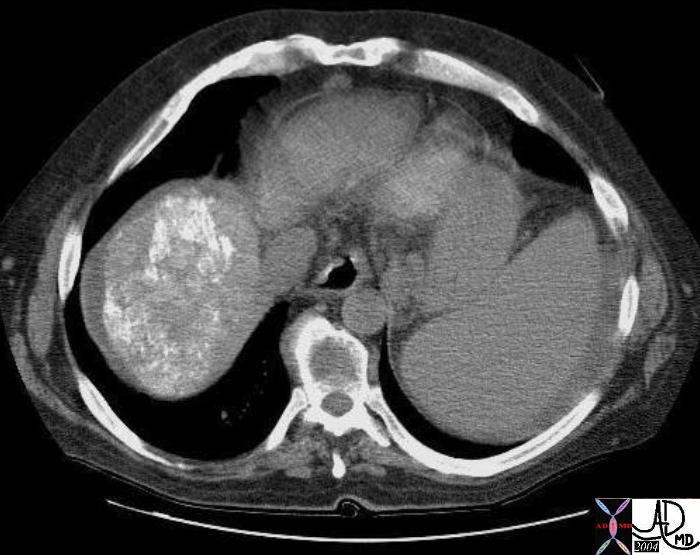
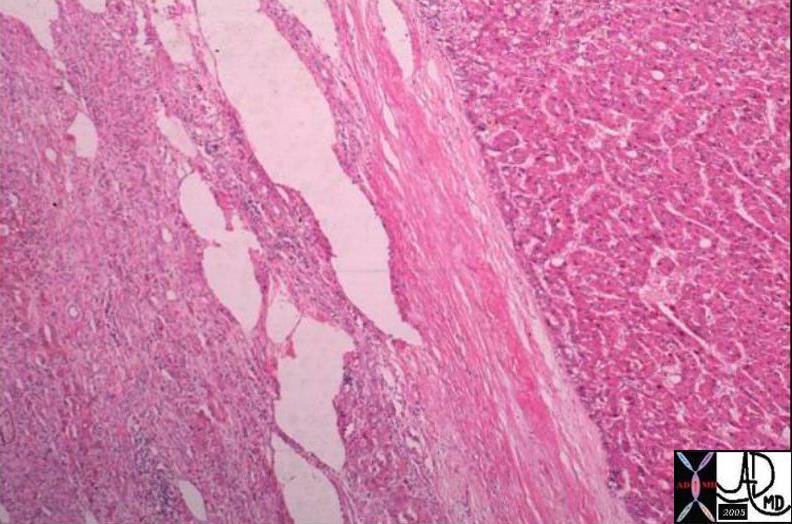
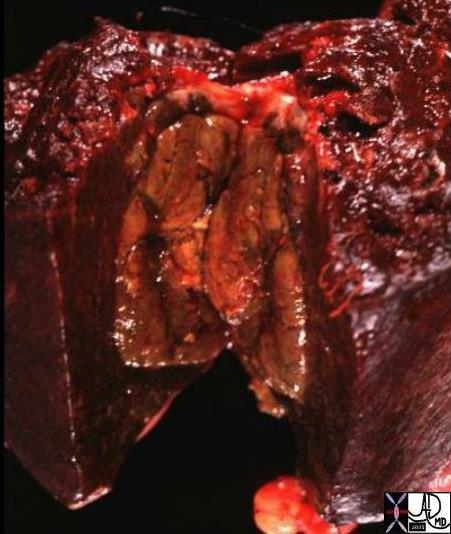
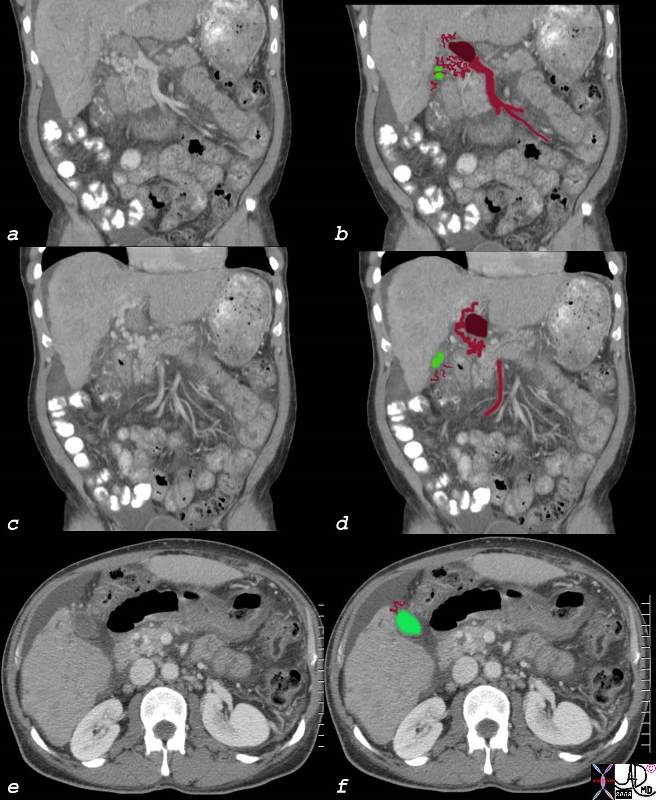

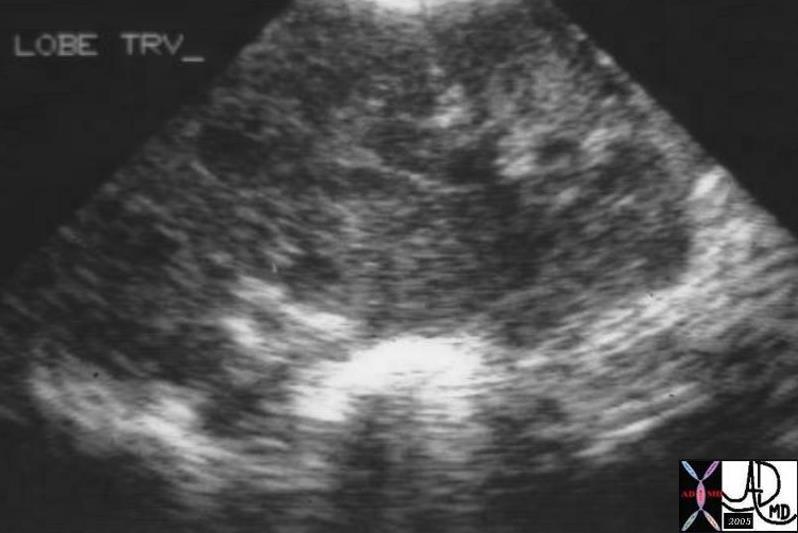
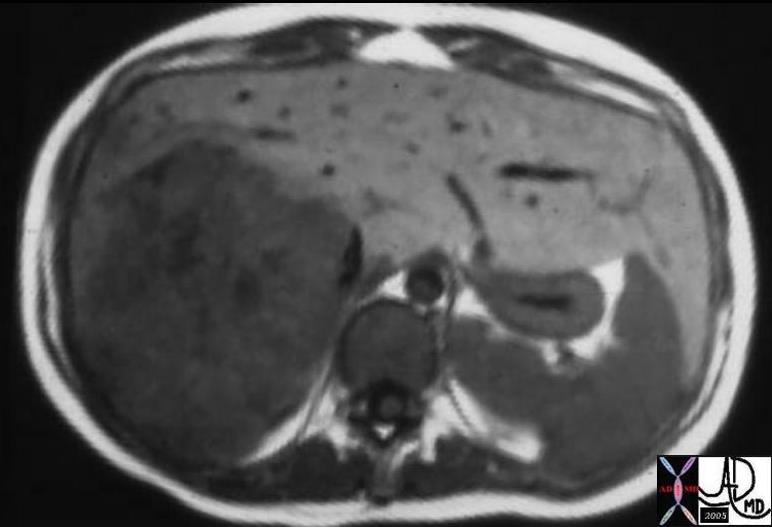
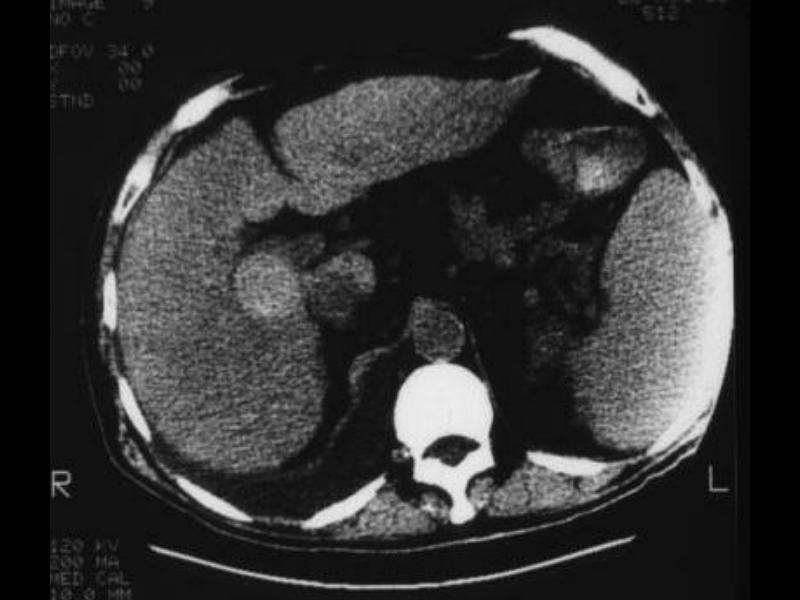
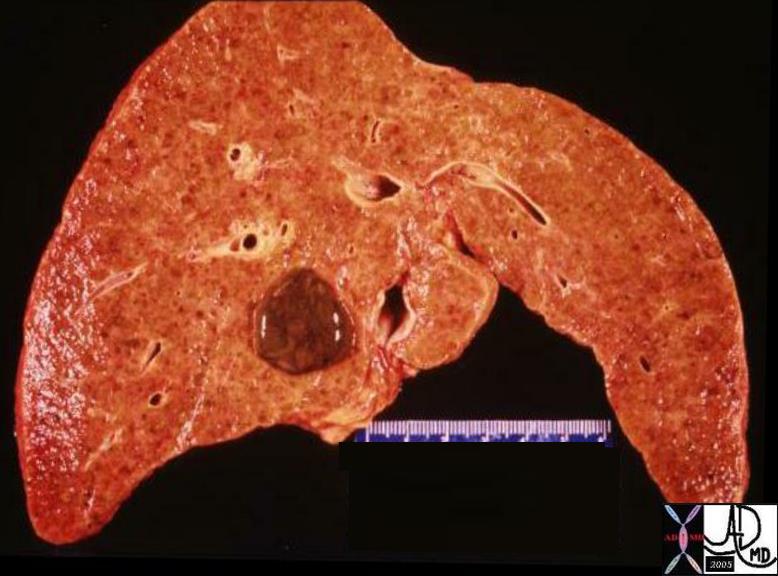
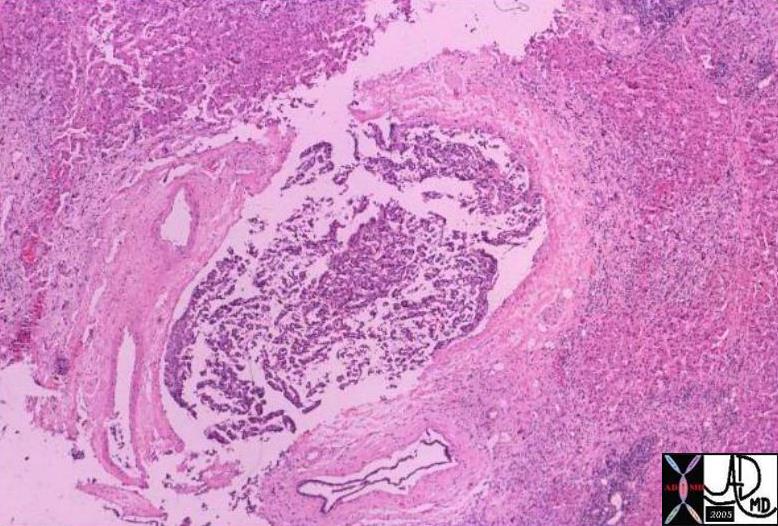




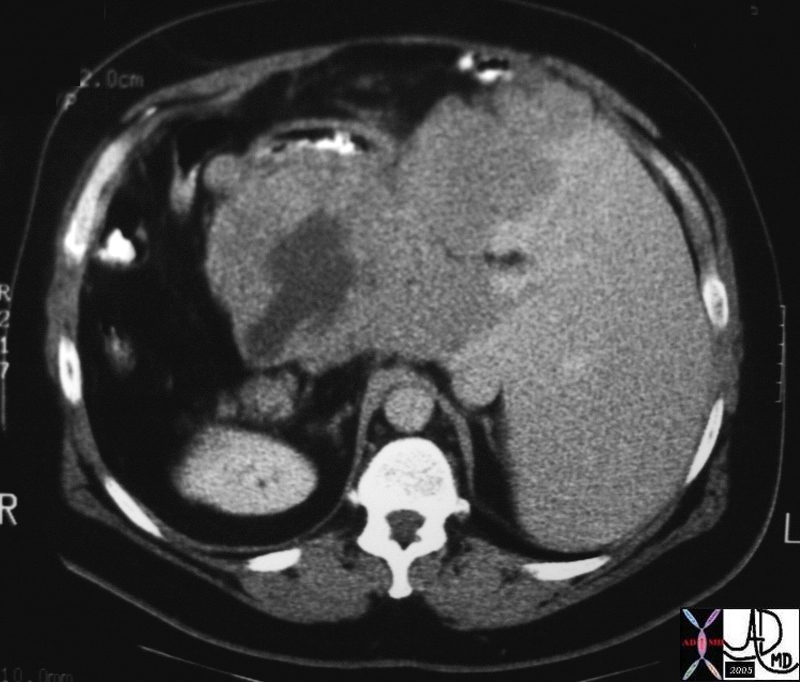
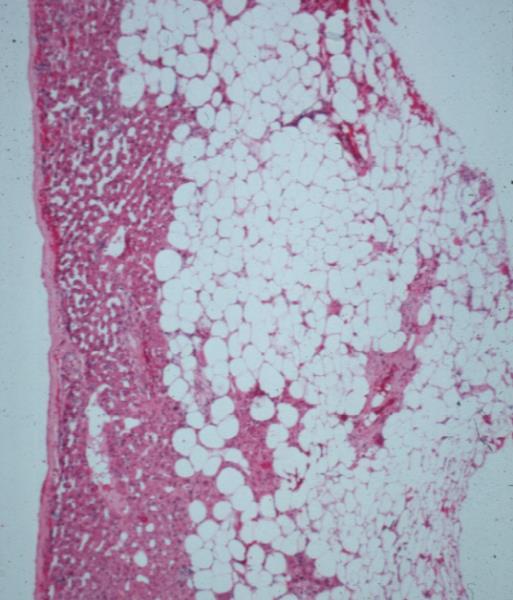
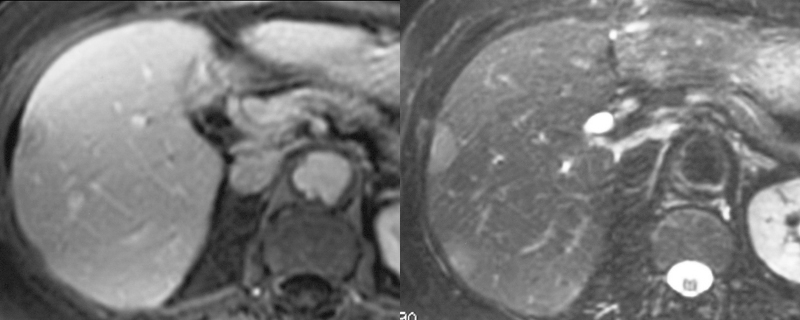
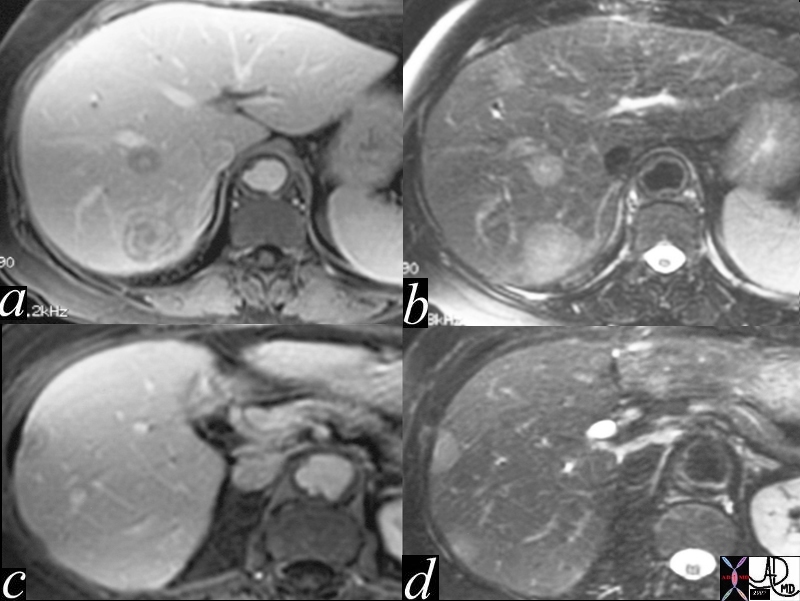
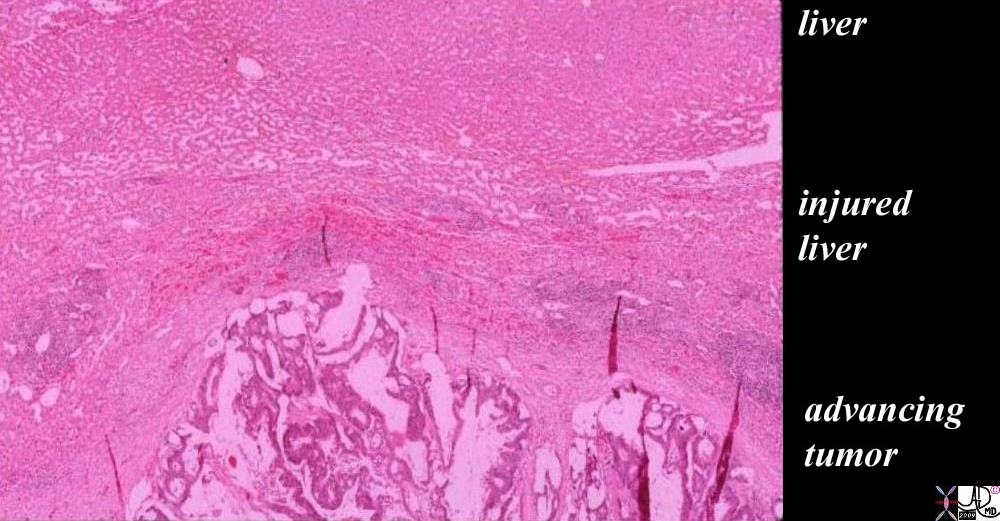
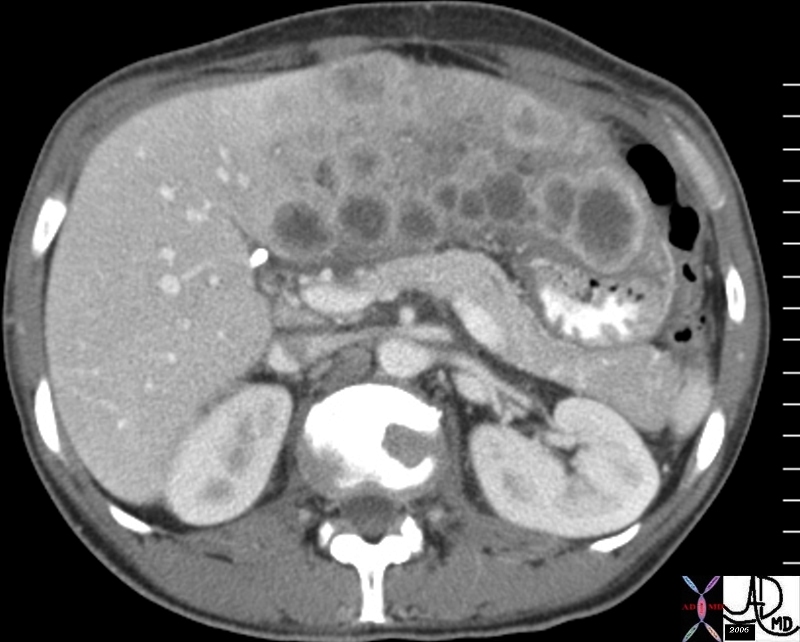
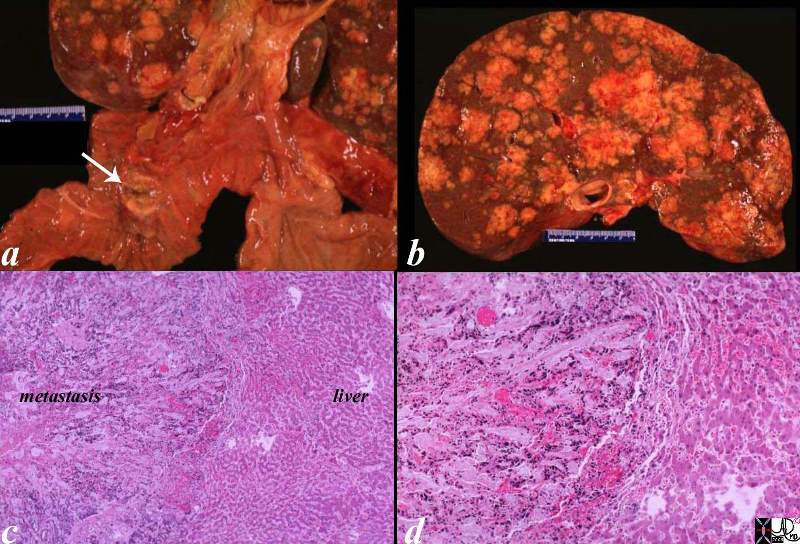
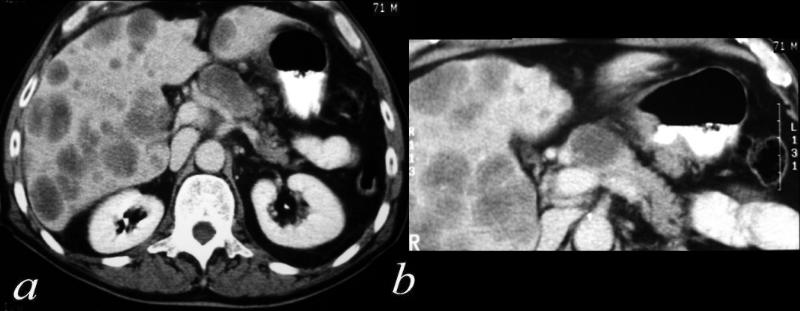
 02891c03.8s
02891c03.8s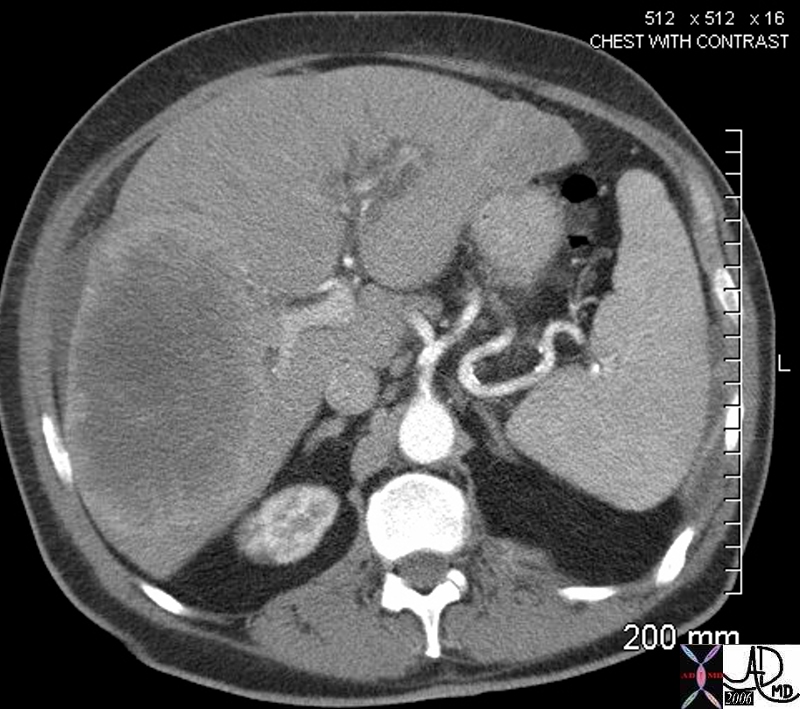 45220
45220
 02986.8s
02986.8s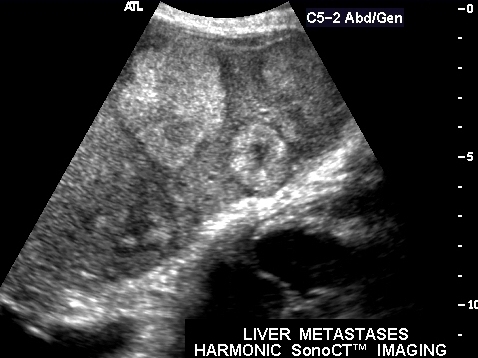
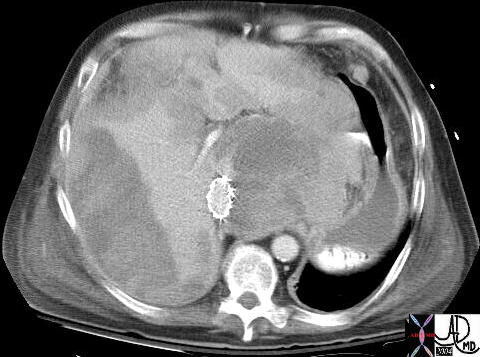
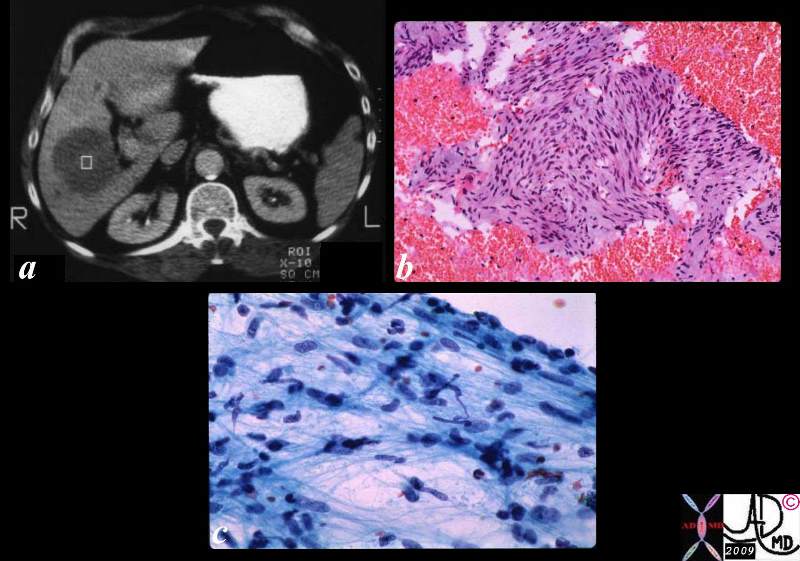
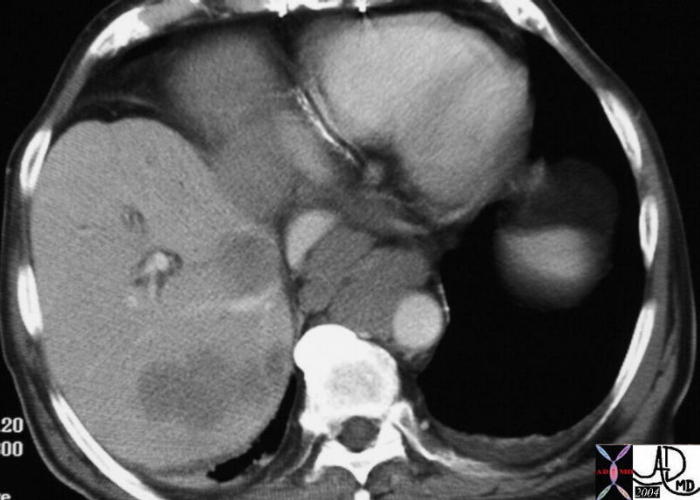
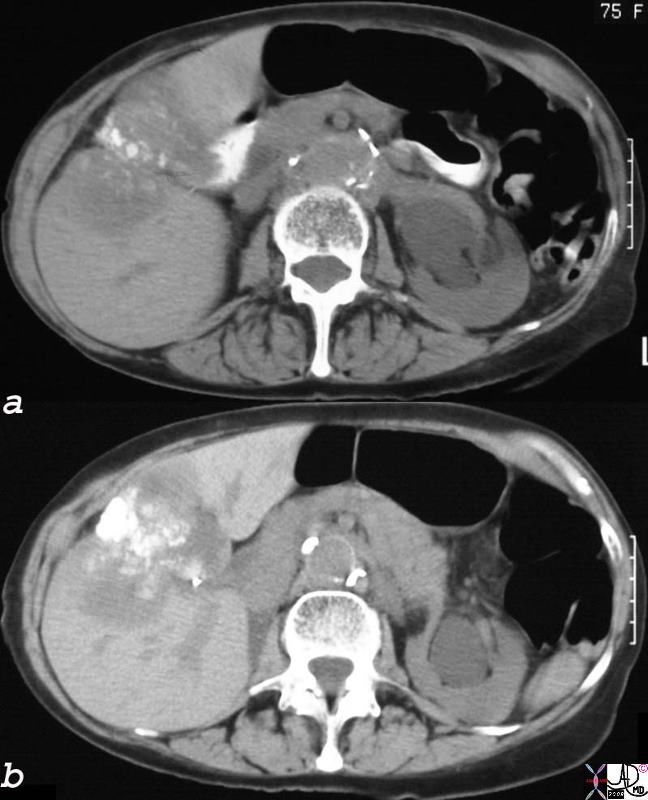
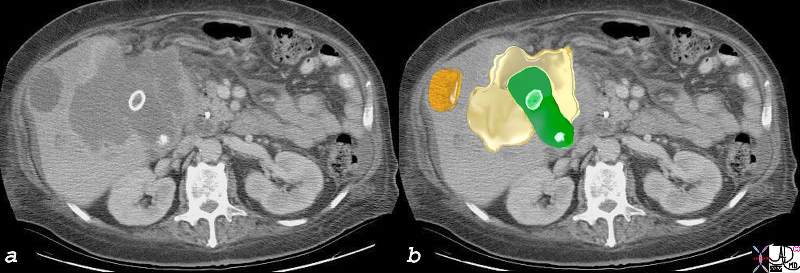
 Breast Carcinoma
Breast Carcinoma
 Components of the Breast
Components of the Breast




 Microscopic Histology of the Lobule
Microscopic Histology of the Lobule Spiculated Carcinoma
Spiculated Carcinoma Soft Tissue Mass – Invasive Ductal Carcinoma
Soft Tissue Mass – Invasive Ductal Carcinoma Invasive Ductal Carcinoma DCIS , Positive Nodes
Invasive Ductal Carcinoma DCIS , Positive Nodes Invasive Scirrhous Ductal Carcinoma
Invasive Scirrhous Ductal Carcinoma Infiltrating Ductal CArcinoma
Infiltrating Ductal CArcinoma Invasive Lobular Carcinoma
Invasive Lobular Carcinoma MRI – Invasive Lobular Carcinoma
MRI – Invasive Lobular Carcinoma Ill defined Mass in the Surical Specimen
Ill defined Mass in the Surical Specimen
 Histopathology of Invasive Lobular Carcinoma
Histopathology of Invasive Lobular Carcinoma CT scan – Inflammatory Carcinoma
CT scan – Inflammatory Carcinoma Well Circumscribed Mass – Phyllodes Tumor
Well Circumscribed Mass – Phyllodes Tumor Magnified View of the Well Circumscribed Phyllodes Tumor
Magnified View of the Well Circumscribed Phyllodes Tumor
 Histopathology of the Phyllodes Tumor
Histopathology of the Phyllodes Tumor Well Defined Mass
Well Defined Mass Ultrasound Showing a Cyst
Ultrasound Showing a Cyst Ultrasound Showing a Solid Lesion – Fibroadenoma
Ultrasound Showing a Solid Lesion – Fibroadenoma
 Microcalcifications Identified on Mammography
Microcalcifications Identified on Mammography Specimen Biopsy Confirms That Correct Tissue has Been Biopsied
Specimen Biopsy Confirms That Correct Tissue has Been Biopsied
 Cytopathology Specimen Infiltrating Ductal Carcinoma
Cytopathology Specimen Infiltrating Ductal Carcinoma Lymphatic supply of the breast
Lymphatic supply of the breast
 Lymph nodes
Lymph nodes